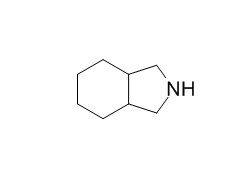
Octahydroisoindole
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Foods.2022, 11(12):1708.
Natural Product Sciences2023, 29(4):276-280.
Nutrients.2024, 16(16):2612.
Revista Brasileira de Farmacognosia2024, 34:1091-1100.
Evidence-based Compl.&Alternative Med.2023, 5417813
Antioxidants (Basel).2020, 9(2): E119
Food and Fermentation Industries2018, 44(371)
Int J Mol Sci.2024, 25(12):6456.
Molecules.2024, 29(21):5161.
Biomed Sci Letters.2020, 26:319-326
Related and Featured Products
European Journal of Organic Chemistry, 2011,33:6732–6738.
Synthesis of Phosphoproline Derivatives with an Octahydroisoindole Structure[Reference:
WebLink]
METHODS AND RESULTS:
The synthesis of two phosphoproline analogues possessing an Octahydroisoindole structure is described for the first time. The new α-aminophosphonic acids can be viewed as the result of fusing a cyclohexane ring to the [c] face of the five-membered pyrrolidine unit. The junction of the bicyclic system is cis in both compounds, but they differ in the relative stereochemistry of the cyclohexane and phosphonate moieties. Using phthalimide as a common precursor and following stereodivergent routes, the target α-aminophosphonic acids, (1R*,3aR*,7aS*)- and (1S*,3aR*,7aS*)-Octahydroisoindole-1-phosphonic acids, have been prepared with completestereocontrol and in high overall yields.
CONCLUSIONS:
The structurally related isoindoline-1-phosphonic acid, containing a benzene ring [c]-fused to pyrrolidine, has also been obtained.