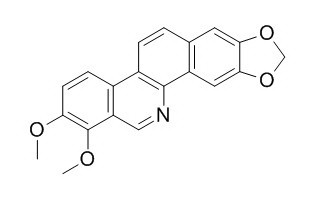
Norchelerythrine
Norchelerythrine shows significant inhibitory activity against Staphylococcus aureus ATCC 6538 with MIC values ranging from 12.5 to 50 ug/mL. It exhibits strong antifeeding activity in a concentration-dependant manner with the EC50 of 62.67 ppm.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Vietnam Journal of Food Control2022, 5(3):pp.390-401.
The Korea Journal of Herbology2020, 35(3):33-45.
Korean Journal of Pharmacognosy.2015, 46(4):352-364
Chung Shan Medical University2020, US20200323790A1
Molecules.2020, 25(21):5087.
Drug Dev Ind Pharm.2024, 50(11):938-951.
Vietnam J. Chemistry2022, 60(2):211-222
Applied Biological Chemistry2021, 64(4)
Mol Cells.2015, 38(9):765-72
Antioxidants (Basel).2020, 9(6):544.
Related and Featured Products
Planta Med. 2012 Jan;78(2):148-53.
Antibacterial Benzofuran Neolignans and Benzophenanthridine Alkaloids from the Roots of Zanthoxylum capense[Pubmed:
22002848 ]
METHODS AND RESULTS:
Two new 2-arylbenzofuran neolignans and a new benzophenanthridine alkaloid, together with six known benzophenanthridine alkaloids, namely, decarine, Norchelerythrine, dihydrochelerythrine, 6-acetonyldihydrochelerythrine, tridecanonchelerythrine, and 6-acetonyldihydronitidine, have been isolated from the MeOH extract of the roots of Zanthoxylum capense. Their structures were elucidated by means of spectroscopic techniques including 2D NMR experiments. All the isolated compounds were evaluated for their in vitro antibacterial activity against gram-positive and gram-negative bacteria.
CONCLUSIONS:
Some compounds showed significant inhibitory activity against Staphylococcus aureus ATCC 6538 with MIC values ranging from 12.5 to 50 μg/mL.
Industrial Crops & Products, 2015 , 74 :407-411.
Antifeedant activities of methanol extracts of four Zanthoxylum species and benzophenanthridines from stem bark of Zanthoxylum schinifolium against Tribolium castaneum[Reference:
WebLink]
METHODS AND RESULTS:
Antifeedant activities of the methanol extracts from Zanthoxylum bungeanum, Zanthoxylum schinifolium, Zanthoxylum armatum and Zanthoxylum dissitum were assessed on Tribolium castaneum adults. It was found that the methanol extract of stem bark of Z. schinifolium had the highest antifeedant activity at 41.12% (antifeedant index). Based on bioactivity-guided fractionation, six benzophenanthridines Norchelerythrine (1), decarine (2), 8-hydroxy-9-methoxy-2,3-(methylenedioxy) benzophenanthridine (3), 6-hydroxydihydrochelerythrine (4), 6-methoxy-7-hydroxydihydrochelerythrine (5) and oxychelerythrine (6) were isolated from the stem barks of Z. schinifolium. And their antifeeding activities were also evaluated against T. castaneum.
All of them exhibited strong antifeeding activity in a concentration-dependant manner with EC50 of 62.67, 66.97, 151.39, 96.72, 141.61 and 192.32 ppm, respectively.
CONCLUSIONS:
The six bioactive compounds from the stem bark of Z. schinifolium might be used as antifeedants against T. castaneum.
Journal of the Chemical Society Perkin Transactions 1.2001 Aug;32(5):523-528.
ChemInform Abstract: Synthesis of Trisphaeridine and Norchelerythrine Through Palladium-Catalyzed Aryl-Aryl Coupling Reactions.[Reference:
WebLink]
METHODS AND RESULTS:
The synthesis of triphaeridine and Norchelerythrine through palladium-catalyzed aryl-aryl coupling reactions was studied. The secondary amides were synthesized from 6-bromopiperonylicacid and aniline, and from piperonylic acid and 2-iodoaniline respectively, and were methoxymethylated for the preparation of starting materials for the cyclization reaction. All experiments were carried out in an argon atmosphere and the extract was washed with brine.
CONCLUSIONS:
The nuclear magnetic resonance (NMR) spectral data showed the presence of singlet signals due to aromatic proton in the products.
Journal of Molecular Structure.2004 Feb 3;689(1–2):115–120.
Theoretical and experimental NMR chemical shifts of norsanguinarine and norchelerythrine.[Reference:
WebLink]
METHODS AND RESULTS:
Norchelerythrine and norsanguinarine, tertiary benzo[c]phenanthridine alkaloids, were examined by gradient-selected 2D NMR spectroscopy and the later also by extensive theoretical calculations. 1H, 13C and 15N chemical shifts assignments of the title isoquinoline alkaloids based on NOE and multiple-bond chemical-shift correlation experiments (GSQMBC) are reported. Various methods were used for the NMR chemical shifts calculations.