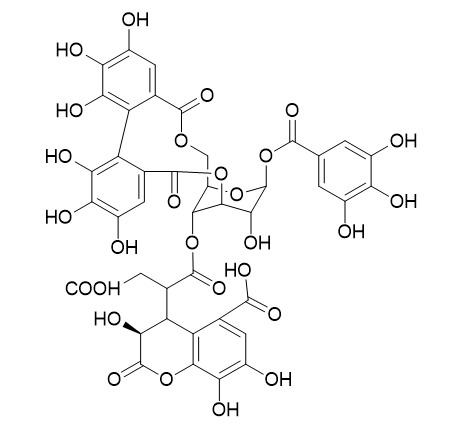
Neochebulagic acid
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Phytother Res.2019, 33(7):1784-1793
Jour. of Stored Pro & Postharvest Res.2016, 7(3):32-36
Food Addit Contam Part A Chem Anal Control Expo Risk Assess.2020, 37(9):1437-1448.
Eur J Pharmacol.2023, 960:176121.
Appl. Sci.2023, 13(17):9984.
Braz J Biol.2023, 82:e266573.
Antibiotics (Basel).2024, 14(1):8.
Environ Toxicol.2023, tox.23999.
FEBS Lett.2021, 595(20):2608-2615.
FASEB J.2022, 36(7):e22387.
Related and Featured Products
J Agric Food Chem. 2012 Sep 5;60(35):8672-83.
Analysis of hydrolyzable tannins and other phenolic compounds in emblic leafflower (Phyllanthus emblica L.) fruits by high performance liquid chromatography-electrospray ionization mass spectrometry.[Pubmed:
22889097]
Phenolic compounds were extracted from dried emblic leafflower (Phyllanthus emblica L.) fruits with methanol and separated by Sephadex LH-20 column chromatography. The raw extracts and fractions were analyzed with HPLC coupled with diode array UV spectroscopy, electrospray ionization mass spectrometry, and tandem mass spectrometry.
METHODS AND RESULTS:
Mucic acid gallate, mucic acid lactone gallate, monogalloylglucose, gallic acid, digalloylglucose, putranjivain A, galloyl-HHDP-glucose, elaeocarpusin, and chebulagic acid were suggested to be the most abundant compounds in the crude methanol extracts of the fruits. In addition, 144 peaks were detected, of which 67 were tentatively identified mostly as ellagitannins, flavonoids, and simple gallic acid derivatives in the fractions. The results indicated the presence of Neochebulagic acid, isomers of neochebuloyl galloylglucose, chebuloyl neochebuloyl galloylglucose, ellagic acid glycosides, quercetin glycosides, and eriodictyol coumaroyl glycosides in the fruits. The study provides a systematic report of the retention data and characteristics of UV, MS, and MS/MS spectra of the phenolic compounds in the fruits of emblic leafflower.
CONCLUSIONS:
The fruits of two varieties (Ping Dan No 1 and Fruity) from Guangxi Province differed from those of wild Tian Chuan emblic leafflower from Fujian Province in the content and profile of phenolic compounds.