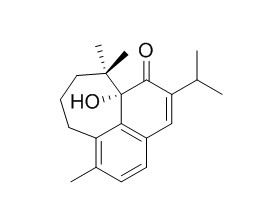
Microstegiol
Migrostegiol has a little activity against B. subtilis.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules.2019, 24(16):E2985
Chem Pharm Bull (Tokyo).2019, 67(11):1242-1247
Plants (Basel).2024, 13(23):3314.
Pharmaceuticals.2022, 15(4), 402.
Int J Biol Macromol.2018, 112:1093-1103
Pharmaceutics.2021, 13(11):1839.
Cell Physiol Biochem.2017, 43(4):1425-1435
Molecules.2018, 23(3):E615
Front Pharmacol.2016, 7:460
Plant Direct.2021, 5(4):e00318.
Related and Featured Products
Fitoterapia. 2005 Jul;76(5):450-2.
Components and antibacterial activity of the roots of Salvia jaminiana.[Pubmed:
15893885 ]
The acetone extract of the roots of Salvia jaminiana, containing the sterols campestanol, stigmasterol and sitosterol, and five known diterpenoids, ferruginol, cryptanol, 6,7-dehydroroyleanon, 6-hydroxysalvinolone and Microstegiol, remarkably inhibited the growth of Bacillus subtilis, Staphylococcus aureus ATCC 25923 and Streptococcus alpha-hemolitic.
Pharmaceutical Biology, 1999 , 37 (2) :148-151.
Cytotoxic Activity of Diterpenoids Isolated from Salvia hypargeia[Reference:
WebLink]
METHODS AND RESULTS:
Ten diterpenoids [6a-hydroxysalvinolone (1), 6�- hydroxytaxodone (2), aethiopinone (3), Microstegiol (4), ferruginol (5), saprorthoquinone (6), 11,12-dioxoabieta-8,13-diene (7), taxodione (8), hypargenin A (9), hypargenin D (10)] and three triterpenoids [lupeol 3- acetate, �-oleanol 3-acetate and ß-sitosterol] were isolated from the roots of Salvia hypargeia, a plant endemic to Turkey. The crude extract and compounds 1-2, 5-10 were tested against a panel of human cancer cell lines [human breast cancer (BC 1), human lung cancer (LU 2), human colon cancer (COL 2), human epidermoidal carcinoma in mouth (KB), vinblastineresistant KB-VI, hormone-dependent human prostate cancer (LNCaP)] as well as P388 and ASK cells in culture.
CONCLUSIONS:
The crude extract was active in all of the test systems, except KB. Isolates 1 and 8 were also found to mediate a generalized cytotoxic response.
Planta Med. 2000 Jun;66(5):458-62.
Antibacterial diterpenes from the roots of Salvia viridis.[Pubmed:
10909268 ]
Three new diterpenes, salviviridinol, viridinol, viridone, five known diterpenes, sugiol, 1-oxoferruginol, ferruginol, aethiopinone and Microstegiol, abietane and rearranged abietane diterpenes were isolated from the roots of Salvia viridis.
METHODS AND RESULTS:
These compounds were assayed against S. aureus ATCC 6538 P, E. coli ATCC 8739, P. mirabilis ATCC 14153, K. pneumonia ATCC 4352, P. aeruginosa ATCC 27853, S. epidermidis ATCC 12228, E. faecalis ATCC 29212 and a yeast C. albicans ATCC 10231.
CONCLUSIONS:
1-Oxoferruginol showed activity against B. subtilis, S. aureus, S. epidermidis and a modest activity against P. mirabilis, migrostegiol had a little activity against B. subtilis. The structures of the compounds were established by 1D and 2D NMR spectroscopic techniques.