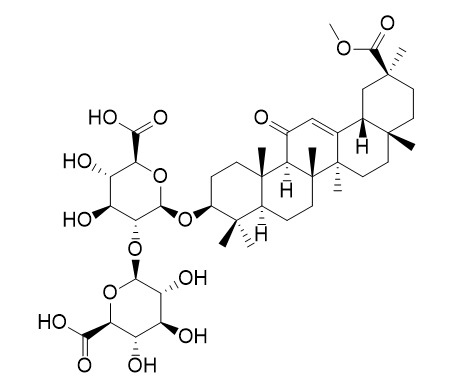
Methyl Glycyrrhizate, derived from Glycyrrhiza uralensis Fisch., is the ester of methyl alcohol and Glycyrrhizic Acid.
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Toxicol . 2007;26 Suppl 2:79-112.
Final report on the safety assessment of Glycyrrhetinic Acid, Potassium Glycyrrhetinate, Disodium Succinoyl Glycyrrhetinate, Glyceryl Glycyrrhetinate, Glycyrrhetinyl Stearate, Stearyl Glycyrrhetinate, Glycyrrhizic Acid, Ammonium Glycyrrhizate, Dipotassium Glycyrrhizate, Disodium Glycyrrhizate, Trisodium Glycyrrhizate, Methyl Glycyrrhizate, and Potassium Glycyrrhizinate[Pubmed:
17613133]
Glycyrrhetinic Acid and its salts and esters and Glycyrrhizic Acid and its salts and esters are cosmetic ingredients that function as flavoring agents or skin-conditioning agents - miscellaneous or both. These chemicals may be isolated from licorice plants. Glycyrrhetinc Acid is described as at least 98% pure, with 0.6% 24-OH-Glycyrrhetinic Acid, not more than 20 mu g/g of heavy metals and not more than 2 mu g/g of arsenic. Ammonium Glycyrrhizate has been found to be at least 98% pure and Dipotassium Glycyrrhizate has been found to be at least 95% pure. Glycyrrhetinic Acid is used in cosmetics at concentrations of up to 2%; Stearyl Glycyrrhetinate, up to 1%; Glycyrrhizic Acid, up to 0.1%; Ammonium Glycyrrhizate, up to 5%; Dipotassium Glycyrrhizate, up to 1%; and Potassium Glycyrretinate, up to 1%. Although Glycyrrhizic Acid is poorly absorbed by the intestinal tract, it may be hydrolyzed to Glycyrrhetinic Acid by a beta -glucuronidase produced by intestinal bacteria. Glycyrrhetinic Acid and Glycyrrhizic Acid bind to rat and human albumin, but do not absorb well into tissues. Glycyrrhetinic Acid and Glycyrrhizic Acid and metabolites are mostly excreted in the bile, with very little excreted in urine. Dipotassium Glycyrrhizate was undetectable in the receptor chamber when tested for transepidermal permeation through pig skin. Glycyrrhizic Acid increased the dermal penetration of diclofenac sodium in rat skin. Dipotassium Glycyrrhizate increased the intestinal absorption of calcitonin in rats. In humans, Glycyrrhetinic Acid potentiated the effects of hydrocortisone in the skin. Moderate chronic or high acute exposure to Glycyrrhizic Acid, Ammonium Glycyrrhizate, and their metabolites have been demonstrated to cause transient systemic alterations, including increased potassium excretion, sodium and water retention, body weight gain, alkalosis, suppression of the renin-angiotensis-aldosterone system, hypertension, and muscular paralysis; possibly through inhibition of 11beta -hydroxysteroid dehydrogenase-2 (11beta -OHSD2) in the kidney. Glycyrrhetinic Acid and its derivatives block gap junction intracellular communication in a dose-dependent manner in animal and human cells, including epithelial cells, fibroblasts, osteoblasts, hepatocytes, and astrocytes; at high concentrations, it is cytotoxic. Glycyrrhetinic Acid and Glycyrrhizic Acid protect liver tissue from carbon tetrachloride. Glycyrrhizic Acid has been used to treat chronic hepatitis, inhibiting the penetration of the hepatitis A virus into hepatocytes. Glycyrrhetinic Acid and Glycyrrhizic Acid have anti-inflammatory effects in rats and mice. The acute intraperitoneal LD(50) for Glycyrrhetinic Acid in mice was 308 mg/kg and the oral LD(50) was > 610 mg/kg. The oral LD(50) in rats was reported to be 610 mg/kg. Higher LD(50) values were generally reported for salts. Little short-term, subchronic, or chronic toxicity was seen in rats given ammonium, dipotassium, or disodium salts of Glycyrrhizic Acid. Glycyrrhetinic Acid was not irritating to shaved rabbit skin, but was considered slightly irritating in an in vitro test. Glycyrrhetinic Acid inhibited the mutagenic activity of benzo[a]pyrene and inhibited tumor initiation and promotion by other agents in mice. Glycyrrhizic Acid inhibited tumor initiation by another agent, but did not prevent tumor promotion in mice. Glycyrrhizic Acid delayed mortality in mice injected with Erlich ascites tumor cells, but did not reduce the mortality rate. Ammonium Glycyrrhizate was not genotoxic in in vivo and in vitro cytogenetics assays, the dominant lethal assay, an Ames assay, and heritable translocation tests, except for possible increase in dominant lethal mutations in rats given 2000 mg/kg day(-1) in their diet. Disodium Glycyrrhizate was not carcinogenic in mice in a drinking water study at exposure levels up to 12.2 mg/kg day(-1) for 96 weeks. Glycyrrhizate salts produced no reproductive or developmental toxicity in rats, mice, golden hamsters, or Dutch-belted rabbits, except for a dose-dependent increase (at 238.8 and 679.9 mg/kg day(-1)) in sternebral variants in a study using rats. Sedation, hypnosis, hypothermia, and respiratory depression were seen in mice given 1250 mg/kg Glycyrrhetinic Acid intraperitoneally. Rats fed a powdered diet containing up to 4% Ammonium Glycyrrhizate had no treatment related effects in motor function tests, but active avoidance was facilitated at 4%, unaffected at 3%, and depressed at 2%. In a study of 39 healthy volunteers, a no effect level of 2 mg/kg/day was determined for Glycyrrhizic Acid given orally for 8 weeks. Clinical tests in seven normal individuals given oral Ammonium Glycyrrhizate at 6 g/day for 3 days revealed reduced renal and thermal sweat excretion of Na+ and K+, but carbohydrate and protein metabolism were not affected. Glycyrrhetinic Acid at concentrations up to 6% was not a skin irritant or a sensitizer in clinical tests. Neither Glycyrrhizic Acid, Ammonium Glycyrrhizate, nor Dipotassium Glycyrrhizate at 5% were phototoxic agents or photosensitizers. Birth weight and maternal blood pressure were unrelated to the level of consumption of Glycyrrhizic Acid in 1049 Finnish women with infants, but babies whose mother consumed > 500 mg/wk were more likely to be born before 38 weeks. The Cosmetic Ingredient Review (CIR) Expert Panel noted that the ingredients in this safety assessment are not plant extracts, powders, or juices, but rather are specific chemical species that may be isolated from the licorice plant. Because these chemicals may be isolated from plant sources, however, steps should be taken to assure that pesticide and toxic metal residues are below acceptable levels. The Panel advised the industry that total polychlorobiphenyl (PCB)/pesticide contamination should be limited to not more than 40 ppm, with not more than 10 ppm for any specific residue, and that toxic metal levels must not contain more than 3 mg/kg of arsenic (as As), not more than 0.002% heavy metals, and not more than 1 mg/kg of lead (as Pb). Although the Panel noted that Glycyrrhizic Acid is cytotoxic at high doses and ingestion can have physiological effects, there is little acute, short-term, subchronic, or chronic toxicity and it is expected that these ingredients would be poorly absorbed through the skin. These ingredients are not considered to be irritants, sensitizers, phototoxic agents, or photosensitizers at the current maximum concentration of use. Accordingly, the CIR Expert Panel concluded that these ingredients are safe in the current practices of use and concentration. The Panel recognizes that certain ingredients in this group are reportedly used in a given product category, but the concentration of use is not available. For other ingredients in this group, information regarding use concentration for specific product categories is provided, but the number of such products is not known. In still other cases, an ingredient is not in current use, but may be used in the future. Although there are gaps in knowledge about product use, the overall information available on the types of products in which these ingredients are used and at what concentration indicate a pattern of use. Within this overall pattern of use, the Expert Panel considers all ingredients in this group to be safe.