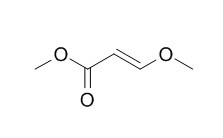
Methyl 3-methoxyacrylate
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
PLoS One.2022, 17(6):e0268505.
Eur J Pharmacol.2018, 832:96-103
J of Advanced Scientific R.2020, 11(3), p109-120.
Oncol Lett.2020, 20(4):122.
Molecules.2023, 28(8):3414.
Int J Mol Sci.2021, 22(17):9400.
Molecules.2021, 26(19):6032.
Pharmaceuticals (Basel).2024 Feb 24;17(3):292.
Phytofrontiers2024, 2690-5442.
Nutr Metab (Lond).2019, 16:31
Related and Featured Products
Organic Process Research & Development, 2002, 6(4):357-366.
Process Research on the Synthesis of Silthiofam: A Novel Fungicide for Wheat.[Reference:
WebLink]
The development of an efficient, low-cost synthesis of the novel wheat fungicide silthiofam (1) is described. Improvements to the original Discovery route allowed 300 kg of material to be prepared in two, overlapping pilot-plant campaigns. Thereafter, efforts were focused on further optimizing the pilot-plant route, and on devising alternate, lower cost routes to silthiofam.
METHODS AND RESULTS:
One potential new route involved a cycloaddition reaction between 3-mercapto-2-butanone and N-(2-propenyl)-3-trimethylsilylpropynamide. The cyclic product could be directly dehydrated to silthiofam, however the overall yield was modest, raw material costs were high, and there were purification problems. The route ultimately selected for development proceeds in 6 chemical steps and about 60% yield from the inexpensive precursors 3-chloro-2-butanone and Methyl 3-methoxyacrylate. Key features of the route are a novel thiophene-3-carboxylate synthesis involving cycloaddition of 3-mercapto-2-butanone with the acrylate followed by acid catalyzed aromatization, the room temperature formation and silylation of a thiophene-3-carboxylate dianion, and conversion of the resulting carboxylic acid into silthiofam with negligible loss of the silyl group.
CONCLUSIONS:
The process involves isolation of just two intermediates, only one of which is purified, and uses only three organic solvents, all of which are recycled. It can be run safely on large scale to give high-purity silthiofam.