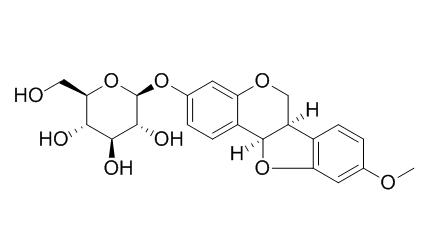
Medicarpin 3-O-glucoside
Medicarpin-3-O-glucoside, a phytoalexin, shows inhibition against the germination of G. intraradices spores and hyphal elongation.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int Immunopharmacol.2019, 71:361-371
Drug Chem Toxicol.2020, 1-12.
Plants (Basel).2022, 11(16):2126.
Food Res Int.2022, 157:111207.
Current Enzyme Inhibition2023, 19(1):55-64(10)
Theranostics.2023, 13(9):3103-3116.
Front Pharmacol.2022, 13:919230.
Food Control2022, 132:108434.
Nutrients.2023, 15(6):1417.
Russian Journal of Bioorganic Chemistry2023, 49:1689¨C1698.
Related and Featured Products
Plant Sci. 2001 Apr;160(5):925-932.
The defense response elicited by the pathogen Rhizoctonia solani is suppressed by colonization of the AM-fungus Glomus intraradices.[Pubmed:
11297789]
METHODS AND RESULTS:
Defense responses of alfalfa roots to the pathogenic fungus Rhizoctonia solani were reduced significantly in roots simultaneously infected with the vesicular arbuscular mycorrhizal (AM) fungus Glomus intraradices. R. solani induced five- to tenfold increases in the steady-state levels of chalcone isomerase and isoflavone reductase mRNAs a doubling of root peroxidase activity and a marked autofluorescence in the infected tissue. These changes were inhibited by the presence of G. intraradices. Interestingly, germination of G. intraradices spores and hyphal elongation were sensitive to low concentrations (2 µM) of Medicarpin 3-O-glucoside, an isoflavonoid phytoalexin that accumulated both in roots colonized by the pathogenic fungus as well as in AM-treated roots receiving high P, where no colonization by the beneficial fungus occurred.
CONCLUSIONS:
These data support the hypothesis that during early stages of colonization by G. intraradices, suppression of defense-related properties is associated with the successful establishment of AM symbiosis.
J Ethnopharmacol. 2018 Jan 30;211:384-393.
Isoflavonoids as wound healing agents from Ononidis Radix.[Pubmed:
28989011 ]
Dried roots of Ononis spinosa L. are traditionally used for their diuretic, anti-inflammatory and wound healing effects. Isolation of the bioactive compounds of Ononis spinosa L. subsp. leiosperma (Boiss.) Sirj.
METHODS AND RESULTS:
Ethyl acetate extract prepared from the roots of Ononis spinosa L. subsp. leiosperma (Boiss.) Sirj. was subjected to silica gel column. The fractions were tested for their wound healing and anti-inflammatory activities. Linear incision and circular excision wound models and hydroxypyroline estimation assay were used for the wound healing activity. Carrageenan-induced hind paw edema, TPA-induced ear edema and acetic acid-induced increase in capillary permeability tests as acute inflammation; FCA-induced arthritis as chronic inflammation models were used for the assessment of anti-inflammatory activity. Antioxidant capacities of the fractions were tested using 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay, 2,2-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid (ABTS) scavenging activity assay, reducing power assay and hydroxyl radical (OH-) scavenging assay. The isolation procedure was continued with the active fraction (Fr-E5). Fr-E5 exhibited remarkable wound healing activity with the 33.4% tensile strength value on the linear incision wound model and 51.4% reduction of the wound area at the day 12 on the circular excision wound model. Hydroxyproline content of the tissue treated by Fr-E5 was found to be 30.9 ± 0.72μg/mg. Acetic acid induced increase in capillary permeability test results revealed that Fr-E5 inhibited inflammation by the value of 40.3%. Fr-E5 showed 28.1-32.2% inhibition in carrageenan-induced hind paw edema test while did not possess activity on TPA-induced ear edema and FCA-induced arthritis models. Trifolirhizin, ononin, Medicarpin 3-O-glucoside, onogenin-7-O-glucoside and sativanone-7-O-glucoside were isolated from Fr-E5 and tested for their wound healing activities using by measuring their inhibition of hyaluronidase, collagenase and elastase enzymes. Ononin and sativanone-7-O-glucoside inhibited hyaluronidase and elastase enzymes by 31.66% and 41.75%; 45.58% and 46.88% values respectively at the dose of 100μg/mL.
CONCLUSIONS:
Among five isolated compounds, ononin and sativanone-7-O-glucoside were found to inhibit hyaluronidase and elastase enzymes. According to the results, these compounds may majorly be responsible for the wound healing activity of the extract.