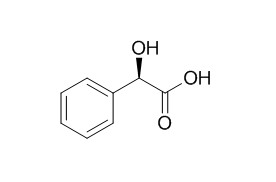
Mandelic acid
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Plant Cell Physiol.2018, 59(1):128-141
Food Addit Contam Part A Chem Anal Control Expo Risk Assess.2020, 37(9):1437-1448.
Applied Biological Chemistry2022, 71:s13765-022-00743-5.
Phytochemistry.2017, 141:162-170
Cells.2022, 11(6):931.
Journal of Cluster Science2024, 35:635-656.
Phytomedicine Plus2024, 4(4): 100655.
J of Archaeological Science:Reports2024, 53:104298
RSC Adv.2024, 14(40):29319-29329.
Sci Adv.2018, 4(10)
Related and Featured Products
Sensors & Actuators B Chemical, 2011, 155(1):140-144.
Enantioselective recognition of chiral mandelic acid in the presence of Zn(II) ions by l -cysteine-modified electrode[Reference:
WebLink]
An obviously enantioselective strategy for the recognition of Mandelic acid (MA) enantiomers in the presence of Zn(II) ions on a l-cysteine ( l-Cys) self-assembled gold electrode is described.
METHODS AND RESULTS:
The high recognition of MA was evaluated via electrochemical impedance spectroscopy and cyclic voltammetry. After the modified electrode interacted with R- or S-MA solution containing Zn(II) ions for 10 min, larger electrochemical response signals were observed for R-MA. Time dependencies of the enantioselective interaction for the modified electrode with the solitary Zn(II) solution and MA enantiomers solutions containing Zn(II) were also investigated.
CONCLUSIONS:
The results showed that the enantioselective recognition was caused by the selective formation of Zn complex with l-Cys and MA enantiomers. In addition, the enantiomeric composition of R- and S-MA enantiomer mixtures could be monitored by measuring the current responses of the sample.