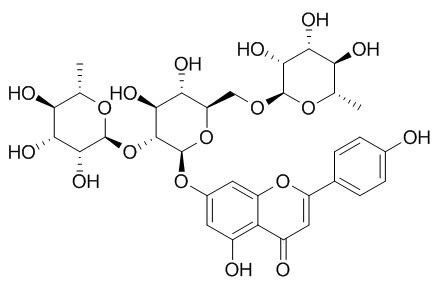
Ligustroflavone
Ligustroflavone shows high antioxidant capacity and is reported to be an AMPK activator, it activates AMPK by increasing ratio of AMP / ATP and promotes adiponectin multimerization by activating AMPK.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
The Thai Journal of Pharmaceutical Sciences2023, 47(3):3.
Current Traditional Medicine, 2021, 7:326-335(10).
Front Plant Sci.2022, 13: 905275.
J Food Sci Technol.2019, 56(5):2712-2720
Biochemical Systematics and Ecology2018, 81
Front Plant Sci.2022, 13:982771.
Eur J Pharmacol.2020, 889:173589.
The Korea Journal of Herbology2020, 35(3):33-45.
Research SPJ.2024, 0377.
Pharmaceutics.2021, 13(7):1028.
Related and Featured Products
J Ethnopharmacol. 2000 Jun;70(3):213-7.
Studies on anti-complementary activity of extracts and isolated flavones from Ligustrum vulgare and Phillyrea latifolia leaves (Oleaceae).[Pubmed:
10837985]
METHODS AND RESULTS:
Polar fractions and flavones isolated from methanolic extracts of the leaves of Ligustrum vulgare and Phillyrea latifolia (Oleaceae), whose popular use as an anti-inflammatory is well-known in Mediterranean historical medicine and ethnobotany, showed significant in vitro complement inhibiting effect on the classical pathway of the complement system.
CONCLUSIONS:
Among the isolated flavonoidic structures, apigenin-7-O-glucoside, apigenin-7-O-rutinoside, luteolin-4'-O-glucoside, luteolin-7-O-glucoside and Ligustroflavone presented remarkable activity.
Research Journal of Phytochemistry, 2014, 8(4):148-154.
Identification of Glycosil Flavones and Determination in vitro of Antioxidant and Photoprotective Activities of Alternanthera brasiliana L. Kuntze.[Reference:
WebLink]
The study Alternanthera brasiliana (L.) Kuntze aims to prove its effectiveness in folk medicine.
METHODS AND RESULTS:
From the study of their chemical constitution were identified by LC-MS technique three glycosylated flavones than were called 2"-O-ramnosylvitexin; 4',5,7-trimethoxy-2"-Oramnosylvitexin and Ligustroflavone. The tests showed high antioxidant capacity of the specie and in small concentrations showed too high levels of photoprotection. In addition its high capacity for photoprotection also paves its use in cosmetology.
CONCLUSIONS:
Alternanthera brasiliana is a species extremely promising with all parameters to become a herbal medicine.
Chinese Journal of Experimental Traditional Medical Formulae, 2016(1):18-21.
Assembly of Adiponectin with Ligustroflavone by Activating AMPK.[Reference:
WebLink]
To investigate activation mechanism of AMPK [adenosine 5'-monophosphate( AMP)-activated protein kinase] by Ligustroflavone,and explore effect of Ligustroflavone on assembly of adiponectin.
METHODS AND RESULTS:
293 T cells were treated with Ligustroflavone. AMPK phosphorylation and evel of cellular AMP and ATP were examined by western blotting and high performance liquid chromatography( HPLC),respectively. 3T3-L1 adipocyte was treated by Ligustroflavone and its effect on assembly of adiponectin was detected. Ligustroflavone can activate AMPK and increase ratio of AMP / ATP in 293 T cells.Ligustroflavone also can promote ssembly of adiponectin multimers.
CONCLUSIONS:
Ligustroflavone activates AMPK by increasing ratio of AMP / ATP and promotes adiponectin multimerization by activating AMPK.
Pharmazie. 2000 Jan;55(1):78-80.
Isolation and structure elucidation of ligustroflavone, a new apigenin triglycoside from the leaves of Ligustrum vulgare L.[Pubmed:
10683879]
A new flavone, apigenin-7-O-beta-(2",6"-di-alpha-rhamnopyranosyl)-glucopyranoside , named Ligustroflavone, was isolated from the leaves of common privet (Ligustrum vulgare L., Oleaceae), whose popular use was well known in the Mediterranean historical medicine and ethnomedicine as anti-inflammatory. The structures of other five apigenin and luteolin derivates, isolated from the polar fractions of the methanolic leaf extracts, were elucidated.
Selina-4(15),7(11)-dien-8-one
Catalog No: CFN95078
CAS No: 54707-47-0
Price: $288/10mg
5,7,2',4'-Tetrahydroxy-8,3'-di(gamma,gamma-dimethylallyl)-isoflavanone
Catalog No: CFN95084
CAS No: 141846-47-1
Price: $413/5mg
Formononetin-8-C-beta-D-apiofuranosyl-(1->6)-O-beta-D-glucopyranoside
Catalog No: CFN95157
CAS No: 1147858-78-3
Price: $318/10mg
Momordicine V
Catalog No: CFN95164
CAS No: 1012315-36-4
Price: $463/5mg
(3E,5E,11E)-tridecatriene-7,9-diyne-1,2-diacetate
Catalog No: CFN95191
CAS No: 94753-06-7
Price: $318/5mg
Isoeuphorbetin
Catalog No: CFN95207
CAS No: 50677-55-9
Price: $318/5mg
Lariciresinol 4'-O-glucoside
Catalog No: CFN95400
CAS No: 107110-16-7
Price: $318/5mg
2,11,12-Trihydroxy-7,20-epoxy-8,11,13-abietatriene
Catalog No: CFN95428
CAS No: 1608462-12-9
Price: $318/10mg
4-O-Coumaroylquinic acid
Catalog No: CFN95508
CAS No: 53539-37-0
Price: $318/5mg
Ganoderic acid beta
Catalog No: CFN95537
CAS No: 217476-76-1
Price: $413/5mg