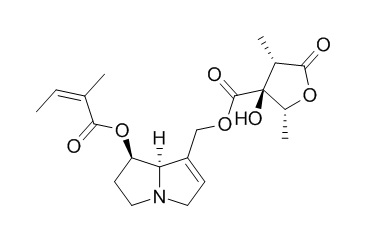
Latifoline
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Mol Sci.2023, 24(7):6360.
Molecules.2020, 25(18):4283.
Asian Pac J Cancer Prev. 2020, 21(4):935-941.
Int J Mol Sci.2021, 22(19):10405.
Korean Herb. Med. Inf.2020, 8(2):243-254.
Journal of Functional Foods2021, 84:104581
Korean Journal of Pharmacognosy.2015, 46(4):352-364
Acta Pharm Sin B.2015, 5(4):323-9.
Oxid Med Cell Longev.2021, 2021:4883398.
Front Pharmacol.2022, 13:906763.
Related and Featured Products
Toxicol Appl Pharmacol. 1993 Sep;122(1):61-9.
Structural influences on pyrrolizidine alkaloid-induced cytopathology.[Pubmed:
8378933]
Pyrrolizidine alkaloids (PAs), which are common constituents of hundreds of plant species around the world, have been reported to have cytotoxic, carcinogenic, antineoplastic, and genotoxic activity in vivo and in vitro. The exact mechanism of these biological toxicities is not yet clear.
METHODS AND RESULTS:
The ability of eight PA congeners to inhibit mitosis and induce megalocyte formation in cultured bovine kidney epithelial cells was studied to examine possible structural influences on these endpoints. Representatives of the three PA structural groups, the macrocycles (seneciphylline, senecionine, riddelliine, retrorsine, monocrotaline), open diesters (heliosupine, Latifoline), and a necine base (retronecine), were cocultured for 2 hr with a NADPH-generating system and rat liver S9. Macrocyclic PAs with alpha,beta-unsaturation (seneciphylline, senecionine, riddelliine, retrorsine) showed a dose-dependent inhibition of colony formation at 50, 100, and 300 microM and induction of megalocytosis at 500 microM. Colony growth resumed 3 weeks after removal of PAs at 50 and 100 microM, and normal cellular morphology returned 5 or 6 weeks after removal of PAs at 500 microM. The saturated macrocyclic (monocrotaline) and open diesters (heliosupine, Latifoline), elicited only a slight inhibition of colony formation and had no effect on cellular morphology at 500 microM. The necine base (retronecine) had no effect on either colony formation or cell morphology. Pyrrolic PAs (dehydrosenecionine, dehydromonocrotaline, dehydroretronecine) were more active in inhibition of colony formation than their parent compounds and were potent inducers of abnormal cellular morphology at 500 microM. An N-oxide metabolite, indicine N-oxide, was completely inactive.
CONCLUSIONS:
The results support previous studies showing that there are structural influences on PA-induced cytopathological effects.
J Org Chem. 2001 Oct 19;66(21):7025-9.
Reactive enols in synthesis 2. Synthesis of (+)-latifolic acid and (+)-latifoline.[Pubmed:
11597224]
METHODS AND RESULTS:
We describe a short, enantioselective synthesis of the naturally occurring pyrrolizidine alkaloid (+)-Latifoline (1) employing a tandem [3,3] sigmatropic rearrangement/[1,2] allyl shift as a key step in constructing (+)-latifolic acid (4).