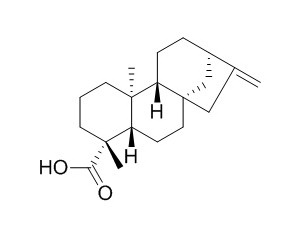
Kaurenoic acid
Kaurenoic acid has anti-inflammatory, analgesic, antimicrobial, cytotoxic and embryotoxic effects; it exerts a uterine relaxant effect acting principally through calcium blockade and in part, by the opening of ATP-sensitive potassium channels. Kaurenoic acid involves the inhibition of cytokine production and activation of the NO-cyclic GMP-protein kinase G-ATP-sensitive potassium channel signaling pathway.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Processes2021, 9(1), 153;
J Pharmacopuncture.2023, 26(4):357-365.
Antioxidants.2022, 11(3):491.
Phytochem Anal.2022, doi: 10.1002
JPC-Journal of Planar Chromatography 2017, 30(2)
Antioxidants (Basel).2019, 8(8):E307
Int J Oncol.2019, 55(1):320-330
Evid Based Complement Alternat Med.2017, 2017:9764843
Biosci Rep.2020, 40(8):BSR20201219.
Natural Product Communications2020, doi: 10.1177.
Related and Featured Products
Evid Based Complement Alternat Med. 2013;2013:160592.
Kaurenoic Acid from Aralia continentalis Inhibits Biofilm Formation of Streptococcus mutans.[Pubmed:
23662113]
We isolated a single chemical compound from A. continentalis and identified it to be Kaurenoic acid (KA) and investigated the influence of anticariogenic properties.
METHODS AND RESULTS:
Inhibitory effects of KA on cariogenic properties such as growth, acid production, biofilm formation, and the adherence of S. mutans were evaluated. Furthermore, real-time PCR analysis was performed to evaluate the influence of KA on the genetic expression of virulence factors. KA significantly inhibited the growth and acid production of S. mutans at 2-4 μ g/mL and 4 μ g/mL of KA, respectively. Furthermore, the adherence onto S-HAs was inhibited at 3-4 μ g/mL of KA and biofilm formation was significantly inhibited when treated with 3 μ g/mL KA and completely inhibited at 4 μ g/mL. Also, the inhibitory effect of KA on biofilm formation was confirmed by SEM. In confocal laser scanning microscopy, bacterial viability gradually decreased by KA in a dose dependent manner. Real-time PCR analysis showed that the expressions of gtfB, gtfC, gbpB, spaP, brpA, relA, and vicR were significantly decreased in S. mutans when it was treated with KA.
CONCLUSIONS:
These results suggest that KA from A. continentalis may be a useful agent for inhibiting the cariogenic properties of S. mutans.
Pharmacol Biochem Behav. 2013 Aug;109:38-43.
Anticonvulsant effect of kaurenoic acid isolated from the root bark of Annona senegalensis.[Pubmed:
23664900]
The herbal preparations of Annona senegalensis Pers. (Annonaceae) root bark are used in Nigerian ethnomedicine for the treatment of epilepsy and febrile seizures. The scientific evidence for this effect has been reported.
The aim of this study was to identify and characterize the active constituent responsible for the anticonvulsant effect.
METHODS AND RESULTS:
Bioactive-guided fractionation of the methanol-methylene chloride root bark extract (MME) of A. senegalensis using pentylenetetrazole (PTZ)-induced seizures in mice, afforded a potent anticonvulsant ethyl-acetate fraction (EF). Further fractionation of the EF yielded eight sub-fractions (F₁-F₈) which were tested for anticonvulsant activity. The sub-fraction F₂ yielded white crystals that were purified to obtain A. senegalensis crystals, AS2. The AS2, which exhibited potent anticonvulsant effects, was characterized by 1D and 2D NMR spectroscopy, mass spectroscopy and X-ray crystallography.
The AS2 was characterized as kaur-16-en-19-oic acid (KA), a diterpenoid. The AS2 indicated an oral LD₅₀ of 3800 mg/kg. The results showed that the MME, EF and AS2 significantly (P<0.05) and dose-dependently delayed the onset of myoclonic spasms and tonic-clonic phases of seizures induced by PTZ and maximal electroshock seizures (MES).
CONCLUSIONS:
Kaurenoic acid was identified as the anticonvulsant principle in the root bark extract of A. senegalensis. The anticonvulsant effect of the MME, EF and AS2 is most likely being mediated through central inhibitory mechanisms.
Phytomedicine. 2011 Jun 15;18(8-9):677-82.
Inhibitory effects of kaurenoic acid from Aralia continentalis on LPS-induced inflammatory response in RAW264.7 macrophages.[Pubmed:
21211951]
This study investigates the anti-inflammatory effects of a diterpenoid, Kaurenoic acid, isolated from the root of Aralia continentalis (Araliaceae).
METHODS AND RESULTS:
To determine its anti-inflammatory effects, LPS-induced RAW264.7 macrophages were treated with different concentrations of Kaurenoic acid and carrageenan-induced paw edema mice model was used in vivo. Kaurenoic acid (ent-kaur-16-en-19-oic acid) dose-dependently inhibited nitric oxide (NO) production, prostaglandin E(2) (PGE(2)) release, cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) expression at micromolar concentrations in LPS-induced RAW264.7 macrophages with IC(50) (the half maximal inhibitory concentration) values of 51.73 (±2.42) μM and 106.09 (±0.27) μM in NO production and PGE(2) release, respectively. Kaurenoic acid also dose-dependently inhibited LPS-induced activation of NF-κB as assayed by electrophorectic mobility shift assay (EMSA) and it almost abolished NF-κB DNA binding affinity at 100μM. Furthermore, the in vivo anti-inflammatory effect of Kaurenoic acid was examined in a carrageenan-induced paw edema model. Eight ICR mice in each group were injected with carrageenan and observed hourly, compared with the control group. Kaurenoic acid dose-dependently reduced paw swelling up to 34.4% at 5h after induction, demonstrating inhibition in an acute inflammation model.
CONCLUSIONS:
Taken together, our data suggest that Kaurenoic acid, a major diterpenoid from the root of A. continentalis shows anti-inflammatory activity and the inhibition of iNOS and COX-2 expression might be one of the mechanisms responsible for its anti-inflammatory properties.
Genet Mol Res. 2013 Apr 2;12(2):1005-11.
The epimer of kaurenoic acid from Croton antisyphiliticus is cytotoxic toward B-16 and HeLa tumor cells through apoptosis induction.[Pubmed:
23613246]
Cancer has become the leading cause of death in developing countries due to increased life expectancy of the population and changes in lifestyle. Studies on active principles of plant have motivated researchers to develop new antitumor agents that are specific and effective for treatment of neoplasms.
Kaurane diterpenes are considered important compounds in the development of new and highly effective anticancer chemotherapeutic agents due to their cytotoxic properties in the induction of apoptosis.
METHODS AND RESULTS:
We evaluated the cytotoxic and apoptotic activity of the epimer of Kaurenoic acid (EKA) isolated from the medicinal plant Croton antisyphiliticus (Euphorbiaceae) toward tumor cell lines HeLa and B-16 and normal fibroblasts 3T3. Based on analyses with the MTT test, EKA showed cytotoxic activity, with half maximal inhibitory concentration values of 59.41, 68.18 and 60.30 μg/mL for the B-16, HeLa and 3T3 cell lines, respectively. The assay for necrotic or apoptotic cells by differential staining showed induction of apoptosis in all three cell lines.
CONCLUSIONS:
We conclude that EKA is not selective between tumor and normal cell lines; the mechanism of action of EKA is induction of apoptosis, which is part of the innate mechanism of cell defense against neoplasia.