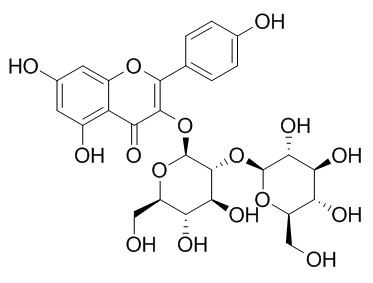
Kaempferol 3-O-beta-sophoroside
Kaempferol 3-O-beta-sophoroside has antibacterial and antiviral activities.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Evid Based Complement Alternat Med.2017, 2017:6360836
ACS Synth Biol.2022, doi: 10.1021.
Nat Prod Sci.2016, 22(2)
Antioxidants (Basel).2020, 9(2):E99
Antioxidants (Basel).2021, 10(11): 1802.
Sustainable Chemistry & Pharmacy2022, 30:100883.
Molecules.2023, 28(4):1526.
ACS Pharmacol. Transl. Sci.2022, 5,7,479-490
Molecules.2021, 26(9):2791.
Biomed Pharmacother.2024, 179:117346.
Related and Featured Products
Inflamm Res . 2012 Mar;61(3):217-24.
Anti-inflammatory effects of kaempferol-3-O-sophoroside in human endothelial cells[Pubmed:
22113342]
Abstract
Background: Kaempferol-3-O-sophoroside (KPOS) was isolated from the leaves of cultivated mountain ginseng. Kaempferol (KP) has antitumor, anti-oxidative, anti-allergic and antidiabetic activities but the barrier protective effects and underlying mechanism are not fully identified. In this study, we attempted to determine whether pretreatment with KPOS induced significant barrier protective activities in lipopolysaccharide (LPS)-stimulated human umbilical vein endothelial cells (HUVECs).
Methods: The anti-inflammatory activities of KPOS were determined by measuring solute flux, neutrophil adhesion and migration and activation of pro-inflammatory proteins in LPS-activated HUVECs.
Results: We found that KPOS inhibited LPS-induced barrier disruption, expression of cell adhesion molecules, neutrophil adhesion and transendothelial migration of neutrophils to HUVECs. Further studies revealed that KPOS suppressed the production of tumor necrosis factor-α (TNF-α) and activation of nuclear factor-κB (NF-κB) by LPS, and that anti-inflammatory activities of KPOS were better than those of KP.
Conclusion: Collectively, these results suggest that KPOS possesses barrier integrity activity, inhibitory activity on cell adhesion and migration to endothelial cells by blocking the activation of NF-κB expression and production of TNF-α, thereby endorsing its usefulness as therapy for vascular inflammatory diseases.
Phytochemistry. 2006 Jan;67(2):191-201.
Flavonols and an indole alkaloid skeleton bearing identical acylated glycosidic groups from yellow petals of Papaver nudicaule.[Pubmed:
16376394]
From yellow petals of Iceland poppy, besides the known flavonoid gossypitrin, seven kaempferol derivatives were isolated.
METHODS AND RESULTS:
In addition to Kaempferol 3-O-beta-sophoroside and Kaempferol 3-O-beta-sophoroside-7-O-beta-glucoside, known from other plants, the mono- and dimalonyl conjugates of the latter were identified by MS and NMR spectroscopy. Structure analyses of a set of co-occurring pigments, the nudicaulins, revealed that they have the identical acylated glycoside moieties attached to a pentacyclic indole alkaloid skeleton for which the structure of 19-(4-hydroxyphenyl)-10H-1,10-ethenochromeno[2,3-b]indole-6,8,18-triol was deduced from MS and NMR as well as chemical and chiroptical methods.