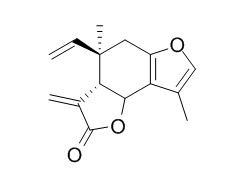
Isolinderalactone
Isolinderalactone shows anti-inflammatory and anticancer capacity, it induces apoptosis in MDA-MB-231 cells and suppresses STAT3 signaling pathway through regulation of SOCS3 and miR-30c, may become a novel treatment for triple-negative breast cancer in the future; it exhibits moderate iNOS inhibitory activity, with the IC50 value of 0.30 uM.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
BioRxiv-The Preprint server for biology2023, 586957.
Hum Exp Toxicol.2023, 42:9603271221145386.
Nutrients.2018, 10(7)
Processes2024, 12(8), 1563
Antiviral Res.2013, 98(3):386-93
Food Science and Biotechnology2022, 10.1007.
Molecules.2020, 25(7):1625.
Toxicol In Vitro.2019, 59:161-178
Plants (Basel).2021, 10(6):1192.
Food Chem.2021, 377:131976.
Related and Featured Products
J Nat Prod. 2011 Dec 27;74(12):2489-96.
Secondary metabolites from the roots of Neolitsea daibuensis and their anti-inflammatory activity.[Pubmed:
22148193]
Bioassay-guided fractionation of the roots of Neolitsea daibuensis afforded three new β-carboline alkaloids, daibucarbolines A-C (1-3), three new sesquiterpenoids, daibulactones A and B (4 and 5) and daibuoxide (6), and 20 known compounds.
METHODS AND RESULTS:
The structures of 1-6 were determined by spectroscopic analysis and single-crystal X-ray diffraction. Daibucarboline A (1), Isolinderalactone (7), 7-O-methylnaringenin (8), and prunetin (9) exhibited moderate iNOS inhibitory activity, with IC₅₀ values of 18.41, 0.30, 19.55, and 10.50 μM, respectively.
Oncol Rep. 2016 Mar;35(3):1356-64.
Isolinderalactone enhances the inhibition of SOCS3 on STAT3 activity by decreasing miR-30c in breast cancer.[Pubmed:
26707189]
Development of an efficient treatment for triple-negative breast cancer is an urgent issues. Compounds from plant extracts are a potential source of novel cancer treatment. Isolinderalactone, a kind of sesquiterpenoids compound, was purified from the root of Lindera strychnifolia and Neolitsea daibuensis and shows anti-inflammatory and anticancer capacity.
METHODS AND RESULTS:
In the present study, Isolinderalactone induced apoptosis in MDA-MB-231 cells which is a kind of triple-negative breast cancer cell line through induction of an intrinsic mitochondria-mediated and caspase-independent cell death. Treatment of Isolinderalactone increased the protein level of the suppressor of cytokine signaling 3 (SCOS3), decreased phosphorylation of the signal transducer and activator of transcription 3 (STAT3), and suppressed expression of the down-stream genes of the X-linked inhibitor of apoptosis protein in MDA-MB-231 cells. Our results further showed that the level of SOCS3 expression was induced by Isolinderalactone due to inhibiting the microRNA hsa-miR-30c-5p (miR-30c) expression. In addition, intraperitoneal injection of Isolinderalactone induced apoptosis in a xenograft breast tumor while it did not significantly affect the histology of liver, kidney and lung of the treated mice.
CONCLUSIONS:
In conclusion, Isolinderalactone induces apoptosis in MDA-MB‑231 cells and suppresses STAT3 signaling pathway through regulation of SOCS3 and miR-30c. It may become a novel treatment for triple-negative breast cancer in the future.
Cancer Lett . 2020 May 28;478:71-81
Isolinderalactone suppresses human glioblastoma growth and angiogenic activity in 3D microfluidic chip and in vivo mouse models[Pubmed:
32173479]
Abstract
Glioblastoma multiforme (GBM) is a lethal and highly vascular type of brain tumor. We previously reported that Isolinderalactone enhances GBM apoptosis in vitro and in vivo, but its role in tumor angiogenesis is unknown. Here, we investigated the anti-angiogenic activity of Isolinderalactone and its mechanisms. In a human GBM xenograft mouse model, Isolinderalactone significantly reduced tumor growth and vessels. Isolinderalactone decreased the expression of vascular endothelial growth factor (VEGF) mRNA, protein, and VEGF secretion in hypoxic U-87 GBM cells and also in xenograft GMB tissue. In addition, we demonstrated that Isolinderalactone significantly inhibited the proliferation, migration, and capillary-like tube formation of human brain microvascular endothelial cells (HBMECs) in the presence of VEGF. We also found that Isolinderalactone decreased sprout diameter and length in a 3D microfluidic chip, and strongly reduced VEGF-triggered angiogenesis in vivo Matrigel plug assay. Isolinderalactone downregulated hypoxia-inducible factor-1α (HIF-1α) and HIF-2α proteins, decreased luciferase activity driven by the VEGF promoter in U-87 cells under hypoxic conditions, and suppressed VEGF-driven phosphorylation of VEGFR2 in HBMECs. Taken together, our results suggest that Isolinderalactone is a promising candidate for GBM treatment through tumor angiogenesis inhibition.
Keywords: 3D microfluidic chip; Angiogenesis; Brain tumor; Hypoxia-inducible factor; Vascular endothelial growth factor.
Mol Med Rep. 2014 May;9(5):1653-9.
Isolinderalactone inhibits proliferation of A549 human non‑small cell lung cancer cells by arresting the cell cycle at the G0/G1 phase and inducing a Fas receptor and soluble Fas ligand-mediated apoptotic pathway.[Pubmed:
24604009]
Lung cancer is currently the leading cause of cancer-related mortality worldwide. In Taiwan, lung cancer is also the type of malignancy that is the major cause of cancer-mortality. Investigating the mechanism of apoptosis of lung cancer cells is important in the treatment of lung cancer.
METHODS AND RESULTS:
In the present study, Isolinderalactone was demonstrated to exhibit anticancer effects in A549 human non-small cell lung cancer cells. The effect of Isolinderalactone on apoptosis, cell cycle distribution p21 levels and the Fas receptor and soluble Fas ligand (sFasL) were assayed in order to determine the mechanism underlying the anticancer effect of Isolinderalactone. It was demonstrated that Isolinderalactone may induce p21 expression and then cause the cell cycle arrest of A549 cells. The data of the present study also revealed that the Fas/sFasL apoptotic system is significant in the mechanism of Isolinderalactone‑induced apoptosis of A549 cells.
CONCLUSIONS:
These novel findings demonstrated that Isolinderalactone may cause the cell cycle arrest of A549 cells by induction of p21, and induce apoptosis of A549 human non-small-cell lung carcinoma cells through the Fas/sFasL apoptotic system.
Chem Cent J. 2013 Jul 30;7(1):131.
Quantitative analysis of the major constituents in Chinese medicinal preparation SuoQuan formulae by ultra fast high performance liquid chromatography/quadrupole tandem mass spectrometry.[Pubmed:
23899222]
The SuoQuan formulae containing Fructus Alpiniae Oxyphyllae has been used to combat the urinary incontinence symptoms including frequency, urgency and nocturia for hundreds of years in China. However, the chemical information was not well characterized. The quality control marker constituent only focused on one single compound in the current Chinese Pharmacopeia. Hence it is prudent to identify and quantify the main constituents in this herbal product. This study aimed to analyze the main constituents using ultra-fast performance liquid chromatography coupled to tandem mass spectrometry (UFLC-MS/MS).
METHODS AND RESULTS:
Fourteen phytochemicals originated from five chemical classes constituents were identified by comparing the molecular mass, fragmentation pattern and retention time with those of the reference standards. A newly developed UFLC-MS/MS was validated demonstrating that the new assay was valid, reproducible and reliable. This method was successfully applied to simultaneously quantify the fourteen phytochemicals. Notably, the content of these constituents showed significant differences in three pharmaceutical preparations. The major constituent originated from each of chemical class was Isolinderalactone, norisoboldine, nootkatone, yakuchinone A and apigenin-4',7-dimethylther, respectively. The variation among these compounds was more than 1000 times. Furthermore, the significant content variation between the two different Suoquan pills was also observed.
CONCLUSIONS:
The proposed method is sensitive and reliable; hence it can be used to analyze a variety of SuoQuan formulae products produced by different pharmaceutical manufacturers.
Yuanhuanin
Catalog No: CFN95127
CAS No: 83133-14-6
Price: $318/5mg
19-O-beta-D-carboxyglucopyranosyl-12-O-beta-D-glucopyranosyl-11,16-dihydroxyabieta-8,11,13-triene
Catalog No: CFN95217
CAS No: 1011714-20-7
Price: $318/5mg
(3R,5S,E)-1,7-Diphenylhept-1-ene-3,5-diol
Catalog No: CFN95227
CAS No: 232261-31-3
Price: $318/5mg
Vanillic acid glucoside
Catalog No: CFN95257
CAS No: 32142-31-7
Price: $368/10mg
Perlolyrine
Catalog No: CFN95291
CAS No: 29700-20-7
Price: $413/5mg
Fuzitine
Catalog No: CFN95337
CAS No: 142287-96-5
Price: $318/5mg
3'-Hydroxy-2,4,5-trimethoxydalbergiquinol
Catalog No: CFN95410
CAS No: N/A
Price: $413/5mg
Kaempferol 3,5-O-diglucoside
Catalog No: CFN95487
CAS No: 205103-97-5
Price: $318/10mg
Ganoderic acid J
Catalog No: CFN95516
CAS No: 100440-26-4
Price: $318/10mg
Methyl ganoderenate F
Catalog No: CFN95573
CAS No: N/A
Price: $413/5mg