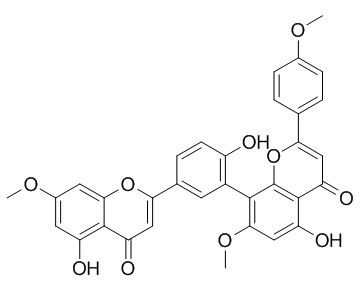
Heveaflavone
Heveaflavone has anti-proliferation effects, it shows moderate Topoisomerase I inhibitory activity. It also possesses a good antioxidant activity via its DPPH free radical scavenging.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Processes2023, 11(2), 385¡£
Molecules.2020, 25(9):2111.
J Appl Biol Chem.2022, 65(4):pp.463-469.
Molecules.2018, 23(3):E615
Plant Pathology2022, 10.1111:ppa.13651.
Evid Based Complement Alternat Med.2018, 2018:4580627
Phytomedicine.2019, 57:95-104
Phytomedicine.2023, 114:154813.
Int J Mol Sci.2024, 25(5):2914.
Antioxidants (Basel).2020, 9(4):284.
Related and Featured Products
Phytochem Anal. 2014 Mar-Apr;25(2):127-33.
Preparative isolation of six anti-tumour biflavonoids from Selaginella doederleinii Hieron by high-speed counter-current chromatography.[Pubmed:
24115163]
Biflavonoids are the primary constituents of Selaginella doederleinii Hieron, to which different bioactivities have been attributed, including anti-cancer, anti-inflammatory, anti-oxidant, anti-fungal and anti-virus activity. However, effective methods for separation of these compounds are not currently available.
To develop a high performance and bioassay-guided method for preparative isolation of biflavonoids from S. doederleini via high-speed counter-current chromatography (HSCCC).
METHODS AND RESULTS:
The anti-proliferation effects of four fractions (70% ethanol, petroleum ether, dichloromethane and acetic ether extracts) of S. doederleinii on five human cancer cells were monitored. The dichloromethane and acetic ether extracts showed good cytotoxicities to the studied cancer cell lines, guiding the subsequent separation. Two solvent systems composed of n-hexane:ethyl acetate:methanol:water (1:2:1.5:1.5, v/v) and n-hexane:ethyl acetate:methanol:water (3:2:3:2, v/v) were developed for separation of the active fractions, respectively. Identification of the biflavonoids was performed by EI-MS(n) , (1) H- and (13) C-NMR.
Under the optimised conditions, 12.6 mg amentoflavone (91.4%), 6.6 mg robustaflavone (90.4%), 7.5 mg 2'', 3''-dihydro-3', 3'''-biapigenin (98.2%) and 7.3 mg 3', 3'''-binaringenin (90.3%) from acetic ether extract (500 mg) and 6.3 mg Heveaflavone (93.5%) and 5.3 mg 7, 4', 7'', 4'''-tetra-O-methyl-amentoflavone (94.5%) from dichloromethane extract (200 mg) were obtained, respectively. The anti-proliferation effects of the six biflavonoids on the five human cancer cells were further verified.
CONCLUSIONS:
The study provides methodological references for simultaneously preparative isolation of several bioactive biflavones from the herbal family of Selaginella. It is the first report discovering 2'', 3''-dihydro-3', 3'''-biapigenin and 3', 3'''-binaringenin from this herb and describing their cytotoxicities to human cancer cell lines.
J Tradit Complement Med. 2012 Jul;2(3):220-6.
Naturally Occurring Cytotoxic [3'→8″]-Biflavonoids from Podocarpus nakaii.[Pubmed:
24716136]
METHODS AND RESULTS:
Bioassay-guided fractionation of the EtOH extract of the dried twigs of Podocarpus nakaii Hayata (Podocarpaceae), endemic plant in Taiwan has resulted in isolation of four [3'→8″]-biflavonoid derivatives, amenotoflavone (AF), podocarpusflavone-A (PF), II-4″,I-7-dimethoxyamentoflavone (DAF), and Heveaflavone (HF). Their structures were determined by physical and extensive spectroscopic analyses such as (1)H, (13)C, (1)H-(1)H COSY, HMQC, and HMBC, as well as comparison with literature values.
CONCLUSIONS:
Compounds PF and DAF showed significant inhibitions against DLD, KB, MCF-7, HEp-2 tumor cell lines (ED50 ca. 4.56-16.24 μg/mL) and induced cell apoptosis in MCF-7 via mainly sub-G1/S phase arrest. Furthermore, these compounds exhibited moderate Topoisomerase I inhibitory activity.
Int J Anal Chem. 2015;2015:849769.
Rapid Screening and Structural Characterization of Antioxidants from the Extract of Selaginella doederleinii Hieron with DPPH-UPLC-Q-TOF/MS Method.[Pubmed:
25792983]
METHODS AND RESULTS:
2,2-Diphenyl-1-picrylhydrazyl-ultra-high performance liquid chromatography-Q-time-of-flight mass spectrometry (DPPH-UPLC-Q-TOF/MS), as a rapid and efficient means, now was used for the first time to screen antioxidants from Selaginella doederleinii.
The nine biflavone compounds were screened as potential antioxidants. The biflavones were structurally identified and divided into the three types, that is, amentoflavone-type, robustaflavone-type, and hinokiflavone-type biflavonoids. Among the compounds bilobetin (3) and putraflavone (8) were found from Selaginella doederleinii for the first time and others including amentoflavone (1), robustaflavone (2), 4'-methoxy robustaflavone (4), podocarpusflavone A (5), hinokiflavone (6), ginkgetin (7), and Heveaflavone (9) were identified previously in the plant. Moreover, nine biflavones possessed a good antioxidant activity via their DPPH free radical scavenging.
CONCLUSIONS:
It demonstrates that DPPH-UPLC-Q-TOF/MS exhibits strong capacity in separation and identification for small molecule. The method is suitable for rapid screening of antioxidants without the need for complicated systems and additional instruments.