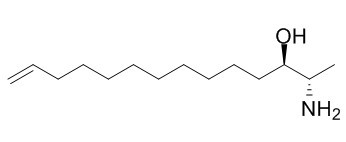
Halaminol A
Halaminol A and haliclonacyclamine A have similar effects on sponge, polychaete, gastropod and bryozoan larvae, inhibit both settlement and metamorphosis, and prevent fouling and colonisation.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Antioxidants (Basel).2021, 10(3):379.
Phytochem Anal.2023, pca.3305.
Biochem Biophys Rep.2024, 40:101830.
Phytomedicine.2018, 41:62-66
J Korean Soc Food Sci Nutr2023, 52(7): 750-757
Mol Pharm.2017, 14(9):3164-3177
Evid Based Complement Alternat Med.2016, 2016:1739760
Drug Des Devel Ther.2023, 17:2461-2479.
J Ethnopharmacol.2020, 249:112381
Oncol Lett.2020, 20(4):122.
Related and Featured Products
Mar Biotechnol (NY). 2009 Mar-Apr;11(2):188-98.
Convergent antifouling activities of structurally distinct bioactive compounds synthesized within two sympatric Haliclona demosponges.[Pubmed:
18690486]
A wide range of sessile and sedentary marine invertebrates synthesize secondary metabolites that have potential as industrial antifoulants. These antifoulants tend to differ in structure, even between closely related species.
METHODS AND RESULTS:
Here, we determine if structurally divergent secondary metabolites produced within two sympatric haliclonid demosponges have similar effects on the larvae of a wide range of benthic competitors and potential fouling metazoans (ascidians, molluscs, bryozoans, polychaetes, and sponges). The sponges Haliclona sp. 628 and sp. 1031 synthesize the tetracyclic alkaloid, haliclonacyclamine A (HA), and the long chain alkyl amino alcohol, Halaminol A (LA), respectively. Despite structural differences, HA and LA have identical effects on phylogenetically disparate ascidian larvae, inducing rapid larval settlement but preventing subsequent metamorphosis at precisely the same stage. HA and LA also have similar effects on sponge, polychaete, gastropod and bryozoan larvae, inhibiting both settlement and metamorphosis. Despite having identical roles in preventing fouling and colonisation, HA and LA differentially affect the physiology of cultured HeLa human cells, indicating they have different molecular targets.
CONCLUSIONS:
From these data, we infer that the secondary metabolites within marine sponges may emerge by varying evolutionary and biosynthetic trajectories that converge on specific ecological roles.
Natural Product Research,2015, 29(24):1-7
Characterisation of the metabolites of an antibacterial endophyte Pat. of L. by LC–MS/MS[Reference:
WebLink]
Botryodiplodia theobromae Pat. belongs to the endophytic fungi that live within the tissues of medicinal plants and produce bioactive natural products.
METHODS AND RESULTS:
The endophyte was isolated from the leaves of Dracaena draco L. The LC-MS-based metabolite fingerprinting of the ethyl acetate extract of B. theobromae with antibacterial activity led to the identification of 13 metabolites pertaining to various classes: dipeptides (maculosin and L,L-cyclo(leucylprolyl), alkaloid (norharman), coumarin and isocoumarins (bergapten, meranzin and monocerin), sesquiterpene (dihydrocumambrin A), aldehyde (formyl indanone), fatty alcohol (Halaminol A) and fatty acid amide (palmitoleamide, palmitamide, capsi-amide and oleamide).
CONCLUSIONS:
This study reports for the first time, the LC-MS and LC-MS/MS identification of 13 known bioactive metabolites from the antibacterial ethyl acetate extract of B.theobromae isolated from the leaves of D. draco L.