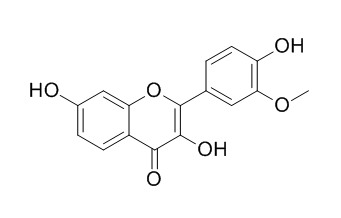
Geraldol
Fisetin is an anticancer agent with antiangiogenic properties in mice, it is reported to suppress endothelial cell migration and proliferation.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Biochem Mol Toxicol.2020, 34(7):e22489.
National Natural Science Foundation of China2024, pages 33.
LWT2021, 138:110397.
J Pharm Biomed Anal.2022, 207:114398.
Antioxidants (Basel).2022, 11(8):1471.
Chin J Appl. Physiol.2019, 35(3):283-288
J Ethnopharmacol.2016, 194:219-227
Appl. Sci.2022, 12(17), 8646.
Anticancer Agents Med Chem.2023, 23(10):1204-1210.
Front Pharmacol.2019, 10:1025
Related and Featured Products
Biochemical Pharmacology, 2011, 82(11):1731-1739.
Fisetin disposition and metabolism in mice: Identification of geraldol as an active metabolite.[Reference:
WebLink]
Although the natural flavonoid fisetin (3,3′,4′,7-tetrahydroxyflavone) has been recently identified as an anticancer agent with antiangiogenic properties in mice, its in vivo pharmacokinetics and metabolism are presently not characterized. Our purpose was to determine the pharmacokinetics and metabolism of fisetin in mice and determine the biological activity of a detected fisetin metabolite.
METHODS AND RESULTS:
After fisetin administration of an efficacious dose of 223 mg/kg i.p. in mice, the maximum fisetin concentration reached 2.5 μg/ml at 15 min and the plasma concentration declined biphasically with a rapid half-life of 0.09 h and a terminal half-life of 3.1 h. Three metabolites were detected, one of which was a glucuronide of fisetin (M1), whereas another glucuronide (M2) was a glucuronide of a previously unknown fisetin metabolite (M3). HPLC–MS/MS analysis indicated that M3 was a methoxylated metabolite of fisetin (MW = 300 Da). The UV spectrum of M3 was identical to that of fisetin and standard 3,4′,7-trihydroxy-3′-methoxyflavone (Geraldol). In addition, because M3 co-eluted with standard Geraldol in 4 different chromatographic ternary gradient conditions, M3 was therefore assigned to Geraldol. Of interest, this metabolite was shown to achieve higher concentrations than fisetin in Lewis lung tumors. We also compared the cytotoxic and antiangiogenic activities of fisetin and Geraldol in vitro and it was found that the latter was more cytotoxic than the parent compound toward tumor cells, and that it could also inhibit endothelial cells migration and proliferation.
CONCLUSIONS:
In conclusion, these results suggest that fisetin metabolism plays an important role in its in vivo anticancer activities.
Drug Metabolism & Pharmacokinetics,2018,33(2):111-117.
Selective inhibition of CYP2C8 by fisetin and its methylated metabolite, geraldol, in human liver microsomes.[Reference:
WebLink]
Fisetin is a flavonol compound commonly found in edible vegetables and fruits. It has anti-tumor, antioxidant, and anti-inflammatory effects. Geraldol, the O-methyl metabolite of fisetin in mice, is reported to suppress endothelial cell migration and proliferation. Although the in vivo and in vitro effects of fisetin and its metabolites are frequently reported, studies on herb–drug interactions have not yet been performed. This study was designed to investigate the inhibitory effect of fisetin and Geraldol on eight isoforms of human cytochrome P450 (CYP) by using cocktail assay and LC-MS/MS analysis.
METHODS AND RESULTS:
The selective inhibition of CYP2C8-catalyzed paclitaxel hydroxylation by fisetin and Geraldol were confirmed in pooled human liver microsomes (HLMs). In addition, an IC50 shift assay under different pre-incubation conditions confirmed that fisetin and Geraldol shows a reversible concentration-dependent, but not mechanism-based, inhibition of CYP2C8. Moreover, Michaelis-Menten, Lineweaver-burk plots, Dixon and Eadie-Hofstee showed a non-competitive inhibition mode with an equilibrium dissociation constant of 4.1 μM for fisetin and 11.5 μM for Geraldol, determined from secondary plot of the Lineweaver-Burk plot.
CONCLUSIONS:
In conclusion, our results indicate that fisetin showed selective reversible and non-competitive inhibition of CYP2C8 more than its main metabolite, Geraldol, in HLMs.