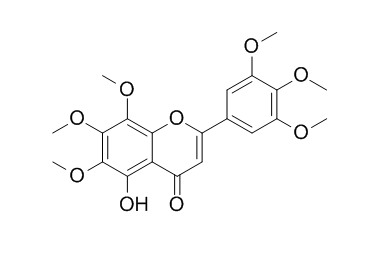
Gardenin A
Gardenin A has antihyperlipidemic and hepatoprotective effects , it also promotes neuritogenesis through the activation of MAPK/ERK-, PKC-, and PKA-dependent, but not TrkA-dependent, CREB signaling pathways in PC12 cells.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
BMC Plant Biol.2021, 21(1):60.
AMB Express2020. 10(1):126.
J Cell Mol Med.2022, 26(23):5807-5819.
Molecules.2024, 29(21):5161.
Biomed Pharmacother.2024, 179:117346.
Cell Death Differ.2021, 1-8.
Pharmaceuticals (Basel).2022, 15(5):591.
Korean Journal of Pharmacognosy2018, 49(3):270-277
Analytical methods2019, 11(6)
Int J Mol Sci.2019, 20(11):E2734
Related and Featured Products
Journal of Agricultural & Food Chemistry, 2013, 61(39):9453-9463.
Neurotrophic Action of 5-Hydroxylated Polymethoxyflavones: 5-Demethylnobiletin and Gardenin A Stimulate Neuritogenesis in PC12 Cells.[Reference:
WebLink]
Polymethoxyflavones (PMFs) exhibit a broad spectrum of biological properties, including anticancer, antiatherogenic, and neuroprotective effects. The aim of this study is to investigate the neurotrophic effects of 5-demethylnobiletin, a hydroxylated PMF found in citrus plants, and Gardenin A, a synthetic PMF analogue, on neurite outgrowth and neuronal differentiation in PC12 cells.
METHODS AND RESULTS:
The results of this study showed that 5-demethylnobiletin and Gardenin A (10–20 μM) potently induce neurite outgrowth in PC12 cells, accompanied by the expression of neuronal differentiation and synapse formation marker proteins, growth-associated protein-43 (GAP-43), and synaptophysin. We observed that the addition of K252a, a TrKA antagonist, significantly inhibited NGF-induced neurite outgrowth in PC12 cells, but 5-demethylnobiletin- or Gardenin A-induced neurite outgrowth was not affected. Treatment with 5-demethylnobiletin and Gardenin A markedly induced the phosphorylation of both cyclic AMP response element-binding protein (CREB) and CRE-mediated transcription, which was suppressed through the administration of the inhibitor 2-naphthol AS-E phosphate (KG-501) or using CREB siRNA. Furthermore, our results showed that MAPK/ERK kinase 1/2 (MEK1/2), protein kinase A (PKA), and protein kinase C (PKC) inhibitors blocked the CRE transcription activity and neurite outgrowth induced through 5-demethylnobiletin or Gardenin A. Consistently, increased ERK phosphorylation and PKA and PKC activities were observed in PC12 cells treated with 5-demethylnobiletin or Gardenin A.
CONCLUSIONS:
These results reveal for the first time that 5-demethylnobiletin and Gardenin A promote neuritogenesis through the activation of MAPK/ERK-, PKC-, and PKA-dependent, but not TrkA-dependent, CREB signaling pathways in PC12 cells.
Chemico Biological Interactions, 2017, 269:9-17.
Antihyperlipidemic and hepatoprotective effects of Gardenin A in cellular and high fat diet fed rodent models.[Reference:
WebLink]
The gum of Gardenia resinifera Roth., is one of the important drugs used in the Indian system of medicine and a source of unique polymethoxylated flavones. This study was aimed to evaluate the antihyperlipidemic and anti-NAFLD effects of Gardenin A (Gar-A) from G. resinifera gum using in vitro and in vivo models. Gar-A was isolated from G. resinifera gum and was identified on the basis of the physical and spectral data.
METHODS AND RESULTS:
Toxicity of Gar-A to HepG2 cells was evaluated using MTT assay. The ability of Gar-A to reduce steatosis was assessed using oleate-palmitate induced HepG2 cell lines by estimating the lipid levels by ORO staining and by estimating the intracellular triglyceride content. Effect of Gar-A on amelioration of lipotoxicity was measured by estimating the LDH levels. The doses for in vivo experiments were fixed by Irwin test, between 50 and 100 mg/kg concentrations, through oral route. The acute antihyperlipidemic effect of Gar-A was assessed in Triton WR–1339 induced hyperlipidemic animals. The chronic antihyperlipidemic and anti-NAFLD effects of Gar-A were evaluated in HFD fed rats. In vitro experiments with HepG2 cell line indicated that the cells treated with Gar-A did not show any significant reduction in the viability up to 70 μg/mL concentration. Steatotic HepG2 cells treated with Gar-A showed a significant reduction in lipid accumulation at 2.5–10 μg/mL concentrations. In triton induced hyperlipidemic rats, the treatment significantly reduced the lipid levels at the synthesis phase.
CONCLUSIONS:
The treatment with Gar-A to the HFD fed animals significantly lowered the steatosis and transaminase levels. The other biochemical parameters such as TC, TG, LDL-c, ALP and ACP were also decreased significantly. Treatment with Gar-A significantly lowered the hyperlipidemia and fat accumulation in the liver; detailed molecular investigations are necessary to establish the antihyperlipidemic and hepatoprotective potentials of Gar-A.