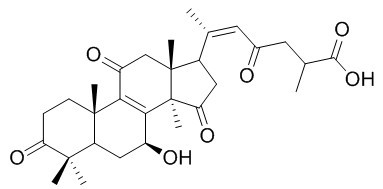
Ganoderenic acid D
Ganoderenic acid D is most cytotoxic with IC50 values of 0.14 ± 0.01, 0.18 ± 0.02 and 0.26 ± 0.03 mg/mL in Hep G2, Hela and Caco-2 cells, respectively.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Comparative Clinical Pathology 2021, 30:961-971.
Food Res Int.2021, 148:110607.
Int J Mol Sci.2020, 21(19),7070.
Biomed Pharmacother.2024, 181:117647.
Oncotarget.2017, 8(53):90925-90947
Nutrients.2024, 16(16):2612.
Fitoterapia.2022, 157:105130.
Heliyon.2023, e12778.
Antioxidants (Basel).2020, 9(7):581.
Biomed Pharmacother.2024, 179:117346.
Related and Featured Products
J Ethnopharmacol . 2018 Jan 10;210:287-295.
Network pharmacology analysis of the anti-cancer pharmacological mechanisms of Ganoderma lucidum extract with experimental support using Hepa1-6-bearing C57 BL/6 mice[Pubmed:
28882624]
Abstract
Ethnopharmacological relevance: Ganoderma lucidum (GL) is an oriental medical fungus, which was used to prevent and treat many diseases. Previously, the effective compounds of Ganoderma lucidum extract (GLE) were extracted from two kinds of GL, [Ganoderma lucidum (Leyss. Ex Fr.) Karst.] and [Ganoderma sinense Zhao, Xu et Zhang], which have been used for adjuvant anti-cancer clinical therapy for more than 20 years. However, its concrete active compounds and its regulation mechanisms on tumor are unclear.
Aim of the study: In this study, we aimed to identify the main active compounds from GLE and to investigate its anti-cancer mechanisms via drug-target biological network construction and prediction.
Materials and methods: The main active compounds of GLE were identified by HPLC, EI-MS and NMR, and the compounds related targets were predicted using docking program. To investigate the functions of GL holistically, the active compounds of GL and related targets were predicted based on four public databases. Subsequently, the Identified-Compound-Target network and Predicted-Compound-Target network were constructed respectively, and they were overlapped to detect the hub potential targets in both networks. Furthermore, the qRT-PCR and western-blot assays were used to validate the expression levels of target genes in GLE treated Hepa1-6-bearing C57 BL/6 mice.
Results: In our work, 12 active compounds of GLE were identified, including Ganoderic acid A, Ganoderenic acid A, Ganoderic acid B, Ganoderic acid H, Ganoderic acid C2, Ganoderenic acid D, Ganoderic acid D, Ganoderenic acid G, Ganoderic acid Y, Kaemferol, Genistein and Ergosterol. Using the docking program, 20 targets were mapped to 12 compounds of GLE. Furthermore, 122 effective active compounds of GL and 116 targets were holistically predicted using public databases. Compare with the Identified-Compound-Target network and Predicted-Compound-Target network, 6 hub targets were screened, including AR, CHRM2, ESR1, NR3C1, NR3C2 and PGR, which was considered as potential markers and might play important roles in the process of GLE treatment. GLE effectively inhibited tumor growth in Hepa1-6-bearing C57 BL/6 mice. Finally, consistent with the results of qRT-PCR data, the results of western-blot assay demonstrated the expression levels of PGR and ESR1 were up-regulated, as well as the expression levels of NR3C2 and AR were down-regulated, while the change of NR3C1 and CHRM2 had no statistical significance.
Conclusions: The results indicated that these 4 hub target genes, including NR3C2, AR, ESR1 and PGR, might act as potential markers to evaluate the curative effect of GLE treatment in tumor. And, the combined data provide preliminary study of the pharmacological mechanisms of GLE, which may be a promising potential therapeutic and chemopreventative candidate for anti-cancer.
Keywords: Cancer; Ergosterol (PubChem CID: 444679); Ganoderenic acid A (PubChem CID: 6442088); Ganoderenic acid D (PubChem CID: 101600080); Ganoderenic acid G (PubChem CID: 14193981); Ganoderic acid A (PubChem CID: 471002); Ganoderic acid B (PubChem CID: 471003); Ganoderic acid C2 (PubChem CID: 57396771); Ganoderic acid D (PubChem CID: 102004379); Ganoderic acid H (PubChem CID: 471005); Ganoderic acid Y (PubChem CID: 57397445); Ganoderma lucidum; Genistein (PubChem CID: 5281377); Kaemferol (PubChem CID: 5280863); Network pharmacology; Target gene.
Natural Product Research, Volume 28, Number 24, 17 December 2014, pp. 2264-2272(9)
Extraction optimisation and isolation of triterpenoids from Ganoderma lucidum and their effect on human carcinoma cell growth[Reference:
WebLink]
The response surface methodology was used to optimise the extraction conditions of Ganoderma lucidum based on a Box–Behnken design.
METHODS AND RESULTS:
A quadratic model sufficiently simulated the response of ganoderic acid H with a determination coefficient (R 2) of 0.98. The optimal condition for extracting triterpenoids was determined to be 100.00% ethanol at 60.22°C for 6.00 h, under which the yield of the reference triterpenoid ganoderic acid H increased from 0.88 to 2.09 mg/g powder. Following extraction, triterpenoid-enriched fraction was further isolated into 23 fractions, and 7 fractions were identified as ganoderic acids A, B, D, G, H and I and Ganoderenic acid D. Of the seven triterpenoids, Ganoderenic acid D was most cytotoxic with IC50 values of 0.14 ± 0.01, 0.18 ± 0.02 and 0.26 ± 0.03 mg/mL in Hep G2, Hela and Caco-2 cells, respectively. While ganoderic acids A, G and H were relatively non-cytotoxic.
CONCLUSIONS:
The variation of inhibitory effects for these triterpenoids was likely related to their chemical structures.