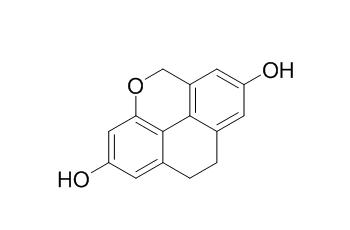
Flavidin
Flavidin shows very good antioxidant capacity. Flavidin can enhance fluorescent imaging, allowing more sensitive and specific cell labeling in tissues, it should have wide application in molecular detection, providing a general insight into how to optimize simultaneously the behavior of the biomolecule and the chemical probe.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Phytomedicine.2023, 116:154841.
Separations2023, 10(4), 231.
Molecules.2020, 25(23):5556.
Molecules.2023, 28(8):3376.
Int J Mol Sci.2022, 23(1):538.
Korean J. Medicinal Crop Sci.2023, 31(6):388-395.
Planta Med.2022, a-1876-3009.
Appl. Sci. 2021, 11(23),11099.
Pharmacol Res.2020, 161:105205.
Naunyn Schmiedebergs Arch Pharmacol.2017, 390(10):1073-1083
Related and Featured Products
PLoS One. 2010 Oct 28;5(10):e13713.
Antioxidant biomarkers from Vanda coerulea stems reduce irradiated HaCaT PGE-2 production as a result of COX-2 inhibition.[Pubmed:
21060890]
METHODS AND RESULTS:
Bio-guided fractionation and phytochemical analysis led to the isolation of five stilbenoids: imbricatin (1) methoxycoelonin (2) gigantol (3) Flavidin (4) and coelonin (5). Stilbenoids (1-3) were the most concentrated in crude hydro-alcoholic stem extract and were considered as Vanda coerulea stem biomarkers.
CONCLUSIONS:
Dihydro-phenanthropyran (1) and dihydro-phenanthrene (2) displayed the best DPPH/(•)OH radical scavenging activities as well as HaCaT intracellular antioxidant properties (using DCFH-DA probe: IC(50) 8.8 μM and 9.4 μM, respectively) compared to bibenzyle (3) (IC(50) 20.6 μM). In turn, the latter showed a constant inhibition of PGE-2 production, stronger than stilbenoids (1) and (2) (IC(50) 12.2 μM and 19.3 μM, respectively).
Western blot analysis revealed that stilbenoids (1-3) inhibited COX-2 expression at 23 μM. Interestingly, stilbenoids (1) and (2) but not (3) were able to inhibit human recombinant COX-2 activity.
Bioorg Med Chem. 2004 Oct 1;12(19):5141-6.
Antioxidant activities of flavidin in different in vitro model systems.[Pubmed:
15351397]
Flavidin was isolated from Orchidaceae species and purified by silica gel column chromatography.
METHODS AND RESULTS:
The structure was identified using physical and spectral ((1)H, (13)C NMR, and mass) data. Antioxidant potency of Flavidin was investigated employing various established in vitro model systems viz., beta-carotene-linoleate, 1,1-diphenyl-2-picryl hydrazyl (DPPH), phosphomolybdenum method, and scavenging of hydrogen peroxide methods. Flavidin showed very good antioxidant activity (90.2%) and almost equivalent to that of BHA at 50ppm level by beta-carotene-linoleate method. Radical scavenging activity of Flavidin was compared with BHA at 5, 10, 20, and 40ppm concentration and Flavidin showed more radical scavenging activity than BHA at all the tested concentrations. Furthermore, Flavidin showed very good antioxidant capacity by the formation of phosphomolybdenum complex method. Besides this, Flavidin showed effective hydrogen peroxide scavenging activity.
CONCLUSIONS:
The data obtained in the in vitro models clearly establish the antioxidant potency of Flavidin. However, comprehensive studies need to be conducted to ascertain the in vivo safety of Flavidin in experimental animal models. This is the first report on antioxidant activity of 9,10-dihydro-5H-phenanthro-(4,5 bcd)-pyrans/Flavidin type of compounds.
Cell Chem Biol. 2017 Aug 17;24(8):1040-1047.e4.
Amine Landscaping to Maximize Protein-Dye Fluorescence and Ultrastable Protein-Ligand Interaction.[Pubmed:
28757182]
Chemical modification of proteins provides great opportunities to control and visualize living systems. The most common way to modify proteins is reaction of their abundant amines with N-hydroxysuccinimide (NHS) esters.
METHODS AND RESULTS:
Here we explore the impact of amine number and positioning on protein-conjugate behavior using streptavidin-biotin, a central research tool. Dye-NHS modification of streptavidin severely damaged ligand binding, necessitating development of a new streptavidin-retaining ultrastable binding after labeling. Exploring the ideal level of dye modification, we engineered a panel bearing 1-6 amines per subunit: "amine landscaping." Surprisingly, brightness increased as amine number decreased, revealing extensive quenching following conventional labeling.
CONCLUSIONS:
We ultimately selected Flavidin (fluorophore-friendly streptavidin), combining ultrastable ligand binding with increased brightness after conjugation. Flavidin enhanced fluorescent imaging, allowing more sensitive and specific cell labeling in tissues. Flavidin should have wide application in molecular detection, providing a general insight into how to optimize simultaneously the behavior of the biomolecule and the chemical probe.