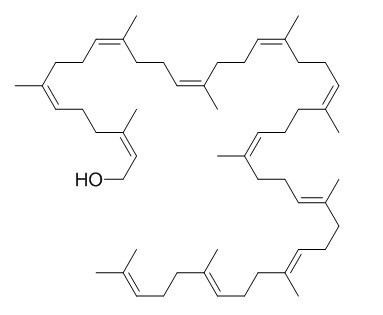
Ficaprenol 11
Standard reference
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Pharmacol Res.2020, 161:105205.
LWT-Food Sci Technol2020, 109163
Int J Mol Sci.2022, 23(20):12516.
Chinese Pharmaceutical Journal2023, 58(2):178-187.
J of Archaeological Science:Reports2024, 53:104298
Int J Mol Sci.2021, 22(9):5012.
Genes Environ.2024, 46(1):13.
Food Science and Preservation2024, 31(3):486-498.
J Food Compos Anal2017, 62:197-204
Phytother Res.2022, ptr.7573.
Related and Featured Products
Biochem J. 1967 Jan;102(1):325-30.
The characterization of ficaprenol-10, -11 and 12 from the leaves of Ficus elastica (decorative rubber plant).[Pubmed:
6030292]
The trivial names ficaprenol 10, Ficaprenol 11 and ficaprenol 12 are proposed.
METHODS AND RESULTS:
Nuclear-magnetic-resonance studies showed that each of these prenols contains three trans internal isoprene residues and a cis ;OH-terminal' isoprene residue. Ficaprenol 11 is the major component of the mixture. Chromatographic evidence suggests the presence also of small amounts of ficaprenol-9 and -13. The precise position of the three trans internal isoprene residues was not determined but it is suggested that these are adjacent to the omega-terminal isoprene residue and that the ficaprenols are formed from all-trans-geranylgeranyl pyrophosphate.
CONCLUSIONS:
It is also suggested that ficaprenol 10, Ficaprenol 11, -12 and -13 are probably the same compounds as castaprenol-10, -11, -12 and -13.
Biochem J. 1979 Oct 1;183(1):163-5.
Structural characterization of ficaprenol-11 by 13C nuclear magnetic resonance.[Pubmed:
534479]
METHODS AND RESULTS:
The location of the internal trans and cis isoprene units in Ficaprenol 11 isolated from Ficus elastica was determined by 13C nuclear magnetic resonance. The alignment of the isoprene units was estimated to be in the order: omega-terminal unit, three trans units, six cis units and alpha-terminal cis alcohol unit.