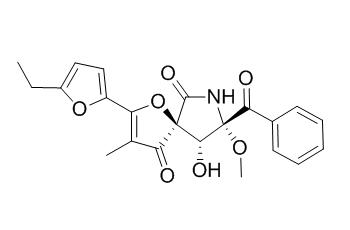
FD-838
FD-838 shows good antileishmanial and moderate anticancer activities, it can moderately inhibit the growth of cultured P388 and HL-60 cell lines. FD-838 has anti-fungal activity, it can significantly inhibit the growth of two plant fungal pathogens Botrytis cinerea and Glomerella cingulata with an minimum inhibitory concentration of 6.25 uM for each, similar to that of the positive fungicide, carbendazim.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Mol Sci.2020, 21(24):9369.
Plant Commun.2024, 5(10):101005.
Evid Based Complement Alternat Med.2016, 2016:1230294
Journal of Oil Palm Research2019, 31(2):238-247
J Biosci.2020, 45:46.
Preprints2022, 202211.0388.v1.
Pharmaceuticals (Basel).2024, 17(1):108.
J of Applied Pharmaceutical Science2020, 10(1):077-082
Phytomedicine.2018, 38:12-23
J Appl Toxicol.2020, 40(7):965-978.
Related and Featured Products
Nat Prod Res. 2014;28(16):1288-92.
Bioactive metabolites from the mycelia of the basidiomycete Hericium erinaceum.[Pubmed:
24635196 ]
METHODS AND RESULTS:
Seven known compounds, three diketopiperazine alkaloids, 12β-hydroxyverruculogen TR-2 (1), fumitremorgin C (2) and methylthiogliotoxin (5), two hetero-spirocyclic γ-lactam alkaloids, pseurotin A (3) and FD-838 (4), and cerevisterol (6) and herierin IV (7), were isolated from the mycelia of the basidiomycete Hericium erinaceum and identified by spectroscopic analyses. The antioxidant and antifungal activities of compounds 1-6 were evaluated.
CONCLUSIONS:
The results indicated that compounds 1, 3 and 6 exhibited potential antioxidant activity against DPPH (2, 2-diphenyl-1-picrylhydrazyl) radical with their IC50 data of ca. 12 μM, compared with positive control tertiary butylhydroquinone. In addition, compound 4 significantly inhibited the growth of two plant fungal pathogens Botrytis cinerea and Glomerella cingulata with an minimum inhibitory concentration of 6.25 μM for each, similar to that of the positive fungicide, carbendazim. Compounds 1-5 were isolated from the genus Hericium for the first time.
Nat Prod Commun. 2012 Feb;7(2):165-8.
Antiparasitic and anticancer constituents of the endophytic fungus Aspergillus sp. strain F1544.[Pubmed:
22474943]
METHODS AND RESULTS:
With the combined goal of finding the best anti-parasitic and anti-cancer activities as well as isolating the bioactive agents and studying their structures and biological properties, we proceeded to perform a small-scale cultivation of Aspergillus sp. strain F1544 using Potato Dextrose, Malt Extract, Czapek Dox and Eight Vegetables media. From the more promising extracts (obtaining using potato dextrose and czapek dox media in large scale) of this fungus, we isolated the five compounds: pseurotin A (1), 14-norpseurotin A (2), FD-838 (3), and pseurotin D (4), and fumoquinone B (5).
CONCLUSIONS:
All compounds showed good antileishmanial and moderate anticancer activities.
J Org Chem. 2010 Jun 18;75(12):4146-53.
The relationship between the CD Cotton effect and the absolute configuration of FD-838 and its seven stereoisomers.[Pubmed:
20507073 ]
METHODS AND RESULTS:
Three new metabolites having a spiro-heterocyclic gamma-lactam core, cephalimysins B-D (1-3), as well as FD-838 (4) were isolated from a culture broth of Aspergillus fumigatus that was originally separated from the marine fish Mugil cephalus. Compounds 1-3 are the diastereomers of 4. Compounds 2 and 3 exhibit an opposite absolute configuration at a spiro carbon to that of other known naturally occurring spiro-heterocyclic gamma-lactams. In addition, we succeeded in the chemical transformation of the four natural products (1-4) into their epimers (1'-4') at C-8 to afford all the stereoisomers of FD-838 (4) with three stereogenic centers.
CONCLUSIONS:
Consequently, the relationship between the absolute configuration at stereogenic centers and the CD Cotton effects for these compounds could be unambiguously established. All of the compounds except 1 moderately inhibited the growth of cultured P388 and HL-60 cell lines.