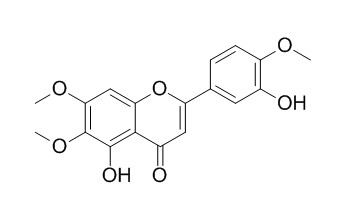
Eupatorin
Eupatorin has antiproliferative and antiangiogenic effects, it emerges as a promising agent in anticancer research.Eupatorin-induced cell death is mediated by both the extrinsic and the intrinsic apoptotic pathways and through a mechanism dependent on reactive oxygen species generation. Eupatorin also has meaningful anti-inflammatory property which may be utilized in the development of novel anti-inflammatory treatments.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Funct Foods2019, 54:449-456
Research Square2021, 10.21203.
Foods.2023, 12(19):3647.
Research on Crops.2017, 18(3):569
J AOAC Int.2024, qsae028.
Environ Toxicol.2022, 37(3):514-526.
Phytomedicine.2024, 129:155645.
Phytomedicine.2024, 125:155350.
Front Nutr.2021, 8: 687851.
Molecules.2017, 22(2)
Related and Featured Products
Fitoterapia. 2012 Sep;83(6):1000-7.
Antiproliferative and antiangiogenic effects of flavone eupatorin, an active constituent of chloroform extract of Orthosiphon stamineus leaves.[Pubmed:
22698713]
Flavone Eupatorin is one of the constituents of Orthosiphon stamineus, a medicinal herb used in folk medicine in South East Asia for treatment of various disorders.
METHODS AND RESULTS:
In our study, we investigated the antiproliferative properties of a chloroform extract of the leaves of O. stamineus and of pure Eupatorin. The compound was able to reduce the number of viable cancer cells to the same extent as the extract, with IC(50) values in micromolar range. Moreover, both the Eupatorin standard and the extract caused cells to arrest in the G2/M phase of the cell cycle. This clearly demonstrates that Eupatorin contributes significantly to the overall extract activity. Induction of mitotic catastrophe, accompanied by key molecular events defining apoptosis, is the mechanism of Eupatorin-induced cell death. Importantly, Eupatorin (at the doses cytotoxic to cancer cells) did not kill normal cells; it only limited migration of HUVEC endothelial cells and their ability to create tubes.
CONCLUSIONS:
The ability of Eupatorin to nonspecifically inhibit many protein kinases was proven and is the probable cause of its cellular effects. In summary, Eupatorin emerges as a promising agent in anticancer research.
Planta Med. 2012 May;78(8):779-86.
Flavonoids eupatorin and sinensetin present in Orthosiphon stamineus leaves inhibit inflammatory gene expression and STAT1 activation.[Pubmed:
22516932]
Cytokines and other inflammatory mediators, such as prostaglandin E₂ (PGE₂) and nitric oxide (NO) produced by cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), respectively, activate and drive inflammation and therefore serve as targets for anti-inflammatory drug development. Orthosiphon stamineus is an indigenous medicinal plant of Southeast Asia that has been traditionally used in the treatment of rheumatoid arthritis, gout, and other inflammatory disorders.
METHODS AND RESULTS:
The present study investigated the anti-inflammatory properties of Orthosiphon stamineus leaf chloroform extract (CE), its flavonoid-containing CE fraction 2 (CF2), and the flavonoids Eupatorin, Eupatorin-5-methyl ether (TMF), and sinensetin, identified from the CF2. It was found that CE (20 and 50 µg/mL) and CF2 (20 and 50 µg/mL) inhibited iNOS expression and NO production, as well as PGE₂ production. Eupatorin and sinensetin inhibited iNOS and COX-2 expression and the production of NO (IC₅₀ 5.2 µM and 9.2 µM for Eupatorin and sinensetin, respectively) and PGE₂ (IC₅₀ 5.0 µM and 2.7 µM for Eupatorin and sinensetin, respectively) in a dose-dependent manner. The extracts and the compounds also inhibited tumor necrosis factor α (TNF-α) production (IC₅₀ 5.0 µM and 2.7 µM for Eupatorin and sinensetin, respectively). Eupatorin and sinensetin inhibited lipopolysaccharide (LPS)-induced activation of transcription factor signal transducers and activators of transcription 1α (STAT1α). Furthermore, Eupatorin (50 mg/kg i. p.) and sinensetin (50 mg/kg i. p.) inhibited carrageenan-induced paw inflammation in mice.
CONCLUSIONS:
The results suggest that CE and CF2, as well as the known constituents of CF2, i.e., Eupatorin and sinensetin, have meaningful anti-inflammatory properties which may be utilized in the development of novel anti-inflammatory treatments.
PLoS One. 2014 Nov 12;9(11):e112536.
Eupatorin-induced cell death in human leukemia cells is dependent on caspases and activates the mitogen-activated protein kinase pathway.[Pubmed:
25390937]
Eupatorin is a naturally occurring flavone that inhibits cell proliferation in human tumor cells.
METHODS AND RESULTS:
Here we demonstrate that Eupatorin arrests cells at the G2-M phase of the cell cycle and induces apoptotic cell death involving activation of multiple caspases, mitochondrial release of cytochrome c and poly(ADP-ribose) polymerase cleavage in human leukemia cells. This flavonoid induced the phosphorylation of members of the mitogen-activated protein kinases and cell death was attenuated by inhibition of c-jun N-terminal kinases/stress activated protein kinases.
CONCLUSIONS:
Eupatorin-induced cell death is mediated by both the extrinsic and the intrinsic apoptotic pathways and through a mechanism dependent on reactive oxygen species generation.
Breast Cancer Res. 2008;10(3):R39.
Antiproliferative and cytostatic effects of the natural product eupatorin on MDA-MB-468 human breast cancer cells due to CYP1-mediated metabolism.[Pubmed:
18454852]
The natural product Eupatorin has been reported to have antiproliferative activity in tumour cell lines, but the exact mechanism is unclear. We aimed to identify a possible mechanism of action for the antiproliferative effect of Eupatorin, which can be attributed to CYP1 family-mediated metabolism.
METHODS AND RESULTS:
The study focuses on the antiproliferative action of Eupatorin on the human breast carcinoma cell line MDA-MB-468 and on a cell line derived from normal mammary tissue, MCF-10A. The cytotoxicity of the flavone, its effect on the cell cycle of the abovementioned cell lines, and its metabolism by CYP1 family enzymes were examined. Eupatorin showed a dose-dependent inhibitory effect of cell growth on MDA-MB-468 cells with a submicromolar median inhibition concentration (IC50) whereas the IC50 of this compound in MCF-10A cells was considerably higher. Moreover, CYP1 family enzymes were shown to metabolise Eupatorin in vitro to the flavone cirsiliol and two other unidentified metabolites. Metabolism of Eupatorin was also detected in MDA-MB-468 cell cultures, whereas metabolism by MCF-10A cells was negligible. Eupatorin was further shown to arrest the cell cycle of the CYP1-expressing cell line MDA-MB-468 in G2/M phase, whereas no effect was observed in MCF-10A cells, which do not express CYP1 enzymes. The effect of Eupatorin on the MDA-MB-468 cell cycle could be reversed by co-application of the CYP1 inhibitor acacetin.
CONCLUSIONS:
The flavone Eupatorin is selectively activated in breast cancer cells, but not in normal breast cells, due to CYP1 family metabolism. This provides a basis for selectivity which is desired against breast tumour cells. In this sense, Eupatorin is shown by this study to be a very promising chemopreventative candidate that should be examined further in an in vivo study.
J Acupunct Meridian Stud. 2012 Aug;5(4):176-82.
A simple isocratic HPLC method for the simultaneous determination of sinensetin, eupatorin, and 3'-hydroxy-5,6,7,4'-tetramethoxyflavone in Orthosiphon stamineus extracts.[Pubmed:
22898066 ]
A rapid, specific reversed-phase HPLC method with isocratic elution of acetonitrile: isopropyl alcohol: 20mM phosphate buffer (NaH(2)PO(4)) (30:15:55, v/v) (pH 3.5) at a flow-rate of 1ml/minute, a column temperature of 25°C, and ultraviolet (UV) detection at 340 nm was developed.
METHODS AND RESULTS:
The method was validated and applied for quantification of different types of O stamineus extracts and fractions. The method allowed simultaneous determination of 3'-hydroxy-5,6,7,4'-tetramethoxyflavone, sinensetin, and Eupatorin in the concentration range of 0.03052-250 μg/ml. The limits of detection and quantification, respectively, were 0.0076 and 0.061 μg/ml for 3'-hydroxy-5,6,7,4'-tetramethoxyflavone, 0.0153 and 0.122 μg/ml for sinensetin and 0.0305 and 0.122 μg/ml for Eupatorin. The percent relative standard deviation (% RSD) values for intraday were 0.048-0.368, 0.025-0.135, and 0.05-0.476 for 3'-hydroxy-5,6,7,4'-tetramethoxyflavone, sinensetin, and Eupatorin, respectively, and those for intraday precision were 0.333-1.688, 0.722-1.055, and 0.548-1.819, respectively. The accuracy for intraday were 91.25%-103.38%, 94.32%-109.56%, and 92.85%-109.70% for 3'-hydroxy-5,6,7,4'-tetramethoxyflavone, sinensetin, and Eupatorin, respectively, and those for interday accuracy were 97.49%-103.92%, 103.58%-104.57%, and 103.9%-105.33%, respectively.
CONCLUSIONS:
The method was found to be simple, accurate and precise and is recommended for routine quality control analysis of O stamineus extract containing the three flavonoids as the principle components in the extract.