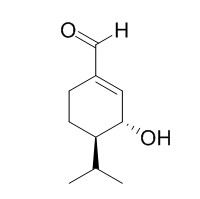
Eucamalol
Eucamolol exhibits significant repellent activity against Aedes albopictus, and inhibits its feeding as well as DEET, is effective repellent (75%) up to 3 h after exposure to mosquito.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules.2022, 27(7):2360.
Anal Bioanal Chem.2023, 415(9):1641-1655.
Journal of Applied Pharmaceutical Science2022, 0(00), pp:001-007
Legume Science2021, 3(4): e101.
J Pharmaceut Biomed2020, 182:113110
Antioxidants (Basel).2021, 10(10):1638.
J Microbiol Biotechnol.2024, 35:e2408022.
Hong Kong Baptist University2023, 048330T.
JMSACL2023, 09.002
Planta Med.2018, 84(15):1101-1109
Related and Featured Products
Biosci Biotechnol Biochem. 1995 Jun;59(6):1139-41.
Absolute configuration of a new mosquito repellent, (+)-eucamalol and the repellent activity of its epimer.[Pubmed:
7613002]
METHODS AND RESULTS:
(+)-Eucamalol (1) and (-)-1-epi-Eucamalol (2) were synthesized from (S)-(-)-perillaldehyde to determine the absolute configuration of 1, the structure of natural (+)-Eucamalol being determined to be (1R,6R)-(+)-3-formyl-6-isopropyl-2-cyclohexen-1-ol. (+)-Eucamolol (1) and its 1-epimer (2) exhibited significant repellent activity against Aedes albopictus, and inhibited its feeding as well as DEET.
J Nat Prod. 2004 Jan;67(1):37-41.
Polyoxygenated eudesmanes and trans-chrysanthemanes from the aerial parts of Santolina insularis.[Pubmed:
14738382]
The eudesmane sesquiterpenoids 1-3 and the trans-chrysanthemyl monoterpenoid 4 have been isolated from the aerial parts of Santolina insularis, a bush endemic to Sardinia.
METHODS AND RESULTS:
The absolute stereostructures of these novel compounds and of two known but incompletely characterized chrysanthemanes (5, 6) were established by spectroscopic techniques and by application of the modified Mosher method. The presence of the p-menthane aldehyde Eucamalol (7) gives credit to the widespread use of S. insularis to fend off mosquitoes.