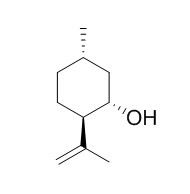
(+)-Isopulegol
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Microbiol Biotechnol.2023, 33(10):1317-1328.
Phytomedicine.2019, 58:152893
Int J Mol Sci.2024, 25(5):2799.
Appl. Sci.2020, 10(23), 8729
Egyptian Pharmaceutical Journal2024, epj_205_23.
Molecules.2022, 27(22):7997.
Pharmaceuticals (Basel).2024, 17(9):1130.
Food Chemistry2023, 137837.
Vietnam J. Chemistry2022, 60(2):211-222
Heliyon.2024, 10(7):e28364.
Related and Featured Products
Chemcatchem, 2017.
Selectivity in the Cyclization of Citronellal Introduced by Squalene Hopene Cyclase Variants.[Reference:
WebLink]
The squalene hopene cyclase from Alicyclobacillus acidocaldarius (AacSHC) is a highly efficient enzyme catalyst for stereoselective Brønsted acid catalysis.
METHODS AND RESULTS:
We engineered AacSHC to catalyze the selective Prins cyclization of citronellal. Four active site variants were identified for the diastereoselective cyclization of (S)‐citronellal to stereoisomers (−)‐iso‐isopulegol,
(+)-Isopulegol and (−)‐neo‐isopulegol, respectively. The replacement of active site residues resulted in two triple variants that catalyzed the transformation of (R)‐citronellal to give the isomers (+)‐neo‐isopulegol and (−)‐isopulegol with up to >99 % de, respectively. The newly designed library of functionally diverse active site geometries exhibits high selective control during citronellal cyclization, leading exclusively to a single diastereomer of the desired isopulegol. Whereas the cyclization of citronellal with chemical catalysts was observed to produce the isopulegol isomer with the lowest energy, the reaction with AacSHC variants proceeded with higher product selectivity.
CONCLUSIONS:
The results of this study show that variants of AacSHC are excellent catalysts for the highly selective formation of isopulegol stereoisomers.