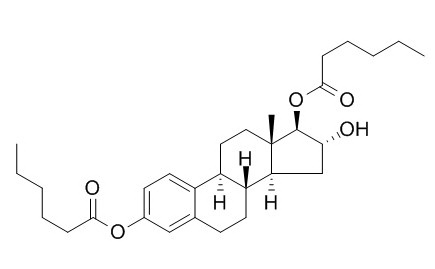
Estriol 3,17-dihexanoate
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Biochem Biophys Res Commun.2017, 482(4):1095-1101
Pathogens.2018, 7(3):E62
Sci Rep.2023, 13(1):13072.
Journal of Plant Growth Regulation2022, 10705-2.
J Lipid Res.2024, 65(10):100640.
Neurochem Int.2020, 133:104629
Mol Med Rep.2024, 29(2):26.
Journal of Food Hygiene and Safety2019, 34(5):413-420
Antibiotics (Basel).2024, 14(1):8.
University of Guelph2021, 12.
Related and Featured Products
Horm Res. 1991;35(6):234-8.
Plasma estriol levels after intramuscular injection of estriol and two of its esters.[Pubmed:
1819548]
METHODS AND RESULTS:
Twelve female volunteers from Berlin and 9 from Stockholm, all using a contraceptive pill (30 micrograms ethinyl estradiol and 150 micrograms levonorgestrel), received an intramuscular injection of estriol (E3; 1 mg in oil) on day 5 of withdrawal bleeding. Blood samples were collected at increasing time intervals during 4 weeks. Three months later, on day 5 of their withdrawal bleeding, 6 women were given intramuscularly (in oil) estriol 3,17-dipropionate (E3-prop) and 15 women Estriol 3,17-dihexanoate (E3-hex). The doses were equivalent to 5 mg of estriol, i.e. 6.94 and 8.90 mg, respectively. Blood samples were collected during a period of 9 weeks. Estriol was analyzed by radioimmunoassay in all plasma samples. The average half-life of E3 ranged from 1.5 to 5.3 h after the administration of E3. It was 12.7 h and between 187 and 221 h after the administration of estriol 3,17-dipropionate and Estriol 3,17-dihexanoate, respectively. The average areas under the curve (in nmol.l-1.h) of E3 were between 82.5 and 161 after the administration of estriol 3,17-dipropionate or Estriol 3,17-dihexanoate, and between 27.1 and 37.9 when E3 had been given. As E3 was administered in a 5-fold lower dose than the esters, the areas under curve appeared to be comparable.
CONCLUSIONS:
Thus, the total exposure to E3 seemed to be almost independent of the type of E3 derivatization, while the time and intensity of exposure were very different.