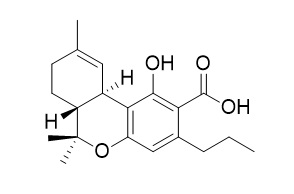
Delta-9-Tetrahydrocannabivarinic acid
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Asian Pac J Tropical Bio.2020, 10(6):239-247
J Ethnopharmacol.2020, 254:112733.
Environ Toxicol.2024, tox.24246
Revista Brasileira de Farmacognosia2024, 34:1091-1100.
Mol Med Rep.2022, 25(1):8.
Dis Markers.2022, 2022:2380879.
Molecules2020, 25(4):892
Biomol Ther (Seoul).2024, 32(5):546-555.
FASEB J.2022, 36(7):e22387.
Molecules 2022, 27(3),1047.
Related and Featured Products
Journal of AOAC International, 2015, 98(6):1503-1522.
Development and validation of a reliable and robust method for the analysis of cannabinoids and terpenes in cannabis.[Reference:
WebLink]
The requirements for an acceptable cannabis assay have changed dramatically over the years resulting in a large number of laboratories using a diverse array of analytical methodologies that have not been properly validated.
METHODS AND RESULTS:
Due to the lack of sufficiently validated methods, we conducted a singlelaboratory validation study for the determination of cannabinoids and terpenes in a variety of commonly occurring cultivars. The procedure involves highthroughput homogenization to prepare sample extract, which is then profiled for cannabinoids and terpenes by HPLC-diode array detector and GC-flame ionization detector, respectively. Spike recovery studies for terpenes in the range of 0.03-1.5% were carried out with analytical standards, while recovery studies for Δ 9 -tetrahydrocannabinolic acid(Delta-9-Tetrahydrocannabivarinic acid), cannabidiolic acid, Δ 9 -tetrahydrocannabivarinic acid, and cannabigerolic acid and their neutral counterparts in the range of 0.3-35% were carried out using cannabis extracts.
CONCLUSIONS:
In general, accuracy at all levels was within 5%, and RSDs were less than 3%. The interday and intraday repeatabilities of the procedure were evaluated with five different cultivars of varying chemotype, again resulting in acceptable RSDs. As an example of the application of this assay, it was used to illustrate the variability seen in cannabis coming from very advanced indoor cultivation operations.