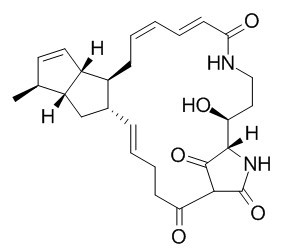
Cylindramide
Cylindramide is cytotoxic against B16 melanoma cells with an IC50 of 0.8 ug/m, it shows promising antiproliferative activity against several tumour cell lines. (+)-Cylindramide has anti-microbial properties.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Appl. Sci.2020, 10(4),1304
Curr Issues Mol Biol.2023, ;45(2):1601-1612.
J Biomol Struct Dyn.2022, 1-21.
Adaptive Medicine 2020, 12(1): 4-10
Natural Product Communications2021, 16(9):1-10.
Translational Neuroscience2024, 15:20220339
Molecules.2024, 29(22):5261.
J Ethnopharmacol.2017, 198:87-90
Biomimetics (Basel).2022, 7(4):154.
Molecules.2023, 28(9):3685.
Related and Featured Products
Tetrahedron Lett., 1993, 34(6):1065-8.
Cylindramide: Cytotoxic tetramic acid lactam from the marine sponge Halichondria cylindrata Tanita & Hoshino[Reference:
WebLink]
METHODS AND RESULTS:
A cytotoxic tetramic acid lactam named Cylindramide (1) has been isolated from the marine sponge Halichondria cylindrata. The structure was determined by interpretation of 2D NMR spectra as a macrocyclic lactam including an acyltetramic acid and a trisubstituted bicyclo[3. 3. 0]octene.
CONCLUSIONS:
This lactam was cytotoxic against B16 melanoma cells with an IC50 of 0.8 μg/mL.
Chembiochem. 2008 Oct 13;9(15):2474-86.
Synthesis and biological properties of cylindramide derivatives: evidence for calcium-dependent cytotoxicity of tetramic acid lactams.[Pubmed:
18798209]
METHODS AND RESULTS:
To gain insight into the biological properties of tetramic acid lactam Cylindramide 1, the analogues 4 a-d bearing a cyclopentane ring instead of the pentalene unit were prepared by tandem conjugate addition/enolate trapping of cyclopentenone 10; a Sonogashira or Stille coupling, followed by a Julia-Kocienski olefination, macrolactamisation and Lacey-Dieckmann cyclisation were the key steps. The previous NMR structure of Cylindramide 1, which was based on NOE and J coupling restraints, could be refined by including residual dipolar coupling data measured for a sample of Cylindramide that was aligned in polyacrylonitrile (18 %). Biological screening of Cylindramide 1 and its analogues 2-epi-1, 20 and 4 revealed promising antiproliferative activity against several tumour cell lines. It turned out that the activity is strongly correlated to the functionalised pentalene system.
CONCLUSIONS:
The configuration of the cyclopentane ring and an intact tetramic acid lactam with the correct configuration seem to play an equal role in the cytotoxicity. The antiproliferative activity was found to be calcium dependent. Phenotypic characterisation of the mode of action showed vacuolisation and vesicle formation in the endoplasmic reticulum.
Chemistry. 2006 Mar 8;12(9):2488-503.
Total synthesis and NMR investigations of cylindramide.[Pubmed:
16389623]
METHODS AND RESULTS:
Cylindramide (1) was built up from three components: a hydroxyornithine derivative 7, a tetrazolylsulfone 8, and a substituted pentalene subunit 9. Derivative 7 was prepared in a six-step reaction sequence involving the Wittig reaction and a Sharpless asymmetric dihydroxylation starting from N-Boc-3-aminopropanal (12).
CONCLUSIONS:
As indicated by extensive 1H and 13C NMR spectroscopic investigations (DQF-COSY, ROESY spectra), the stereochemistry of synthetic Cylindramide (1) corresponds with that of the naturally occurring product. ROE data were used for molecular modeling of the lowest-energy structures for Cylindramide.