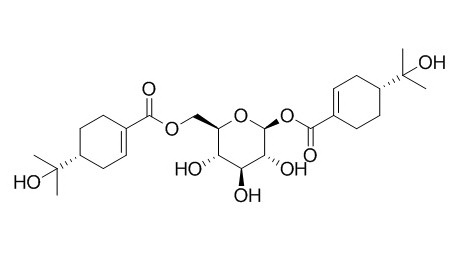
Cuniloside B
Cuniloside B shows anti-leishmanial activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Mol Sci.2024, 25(23):12733.
Talanta Open2023, 7:100227
Pharmacognosy Journal.2022, 14,4,327-337.
Nutr Metab (Lond).2019, 16:31
Natural Product Communications2023, 18(9).
Journal of Apicultural Research2021, 60(1)
Nutrients.2019, 12(1):E40
Front Pharmacol.2022, 13:919230.
Int J Mol Sci.2015, 16(8):18396-411
Food Chemistry: X2023, 101032.
Related and Featured Products
Pharm Biol. 2012 Jul;50(7):823-7.
Terpenoidal constituents of Eucalyptus loxophleba ssp. lissophloia.[Pubmed:
22468852]
Eucalyptus has been a source of a number of biologically active compounds. The anti-leishmanial activity of terpenoids from Eucalyptus loxophleba (Benth.) ssp. lissophloia (Myrtaceae) has not yet been investigated.
Isolation of the terpenoidal constituents for evaluation of in vitro anti-leishmanial activity against the Leishmania donovani (Dd8 strain) promastigotes.
METHODS AND RESULTS:
The chloroform-methanol (8:2) extract of dried leaves of Eucalyptus loxophleba was used to isolate terpenoidal constituents employing solvent partitioning, column chromatography and preparative high performance liquid chromatography and characterized from spectral data. The anti-leishmanial activity of the isolated compounds was tested in vitro using an Alamar blue assay against a culture of L. donovani (Dd8 strain) promastigotes.
Two new naturally occurring triterpenes, named loxanic acid and 3-acetyl loxanic acid together with four known ursane triterpenoids and one bis-monoterpene glycoside, Cuniloside B isolated from the leaves showed anti-leishmanial activity (IC(50) 133 to 235 μM) against the promastigotes of the tested strain.
CONCLUSIONS:
The terpenes isolated from the leaves of E. loxophleba showed moderate anti-leishmanial activity.
Carbohydr Res. 2010 Sep 23;345(14):2079-84.
Synthesis of the monoterpenoid esters cypellocarpin C and cuniloside B and evidence for their widespread occurrence in Eucalyptus.[Pubmed:
20708173]
METHODS AND RESULTS:
Short syntheses of Cuniloside B and cypellocarpin C, (+)-(R)-oleuropeic acid-containing carbohydrates, are reported. Also disclosed are syntheses of the noreugenin glycosides, undulatoside A and corymbosins K(1) and K(2). Leaf extracts of 28 diverse eucalypts revealed Cuniloside B to be present in all, and cypellocarpin C to be present in most, of the species examined.
CONCLUSIONS:
The widespread occurrence of these carbohydrate monoterpenoid esters supports their roles in essential oil biosynthesis or mobilization from sites of synthesis to secretory cavity lumena.