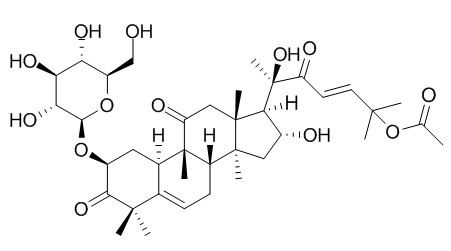
Cucurbitacin B 2-O-beta-D-glucoside
Cucurbitacin B 2-o-β-D-glucoside has anticancer activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Wageningen University & Research2018, January 2018
J Cell Mol Med.2024, 28(16):e70016.
J Korean Med Obes Res.2023, 23:10-7
Molecules.2021, 26(9):2765.
Sci Rep.2025, 15(1):29590.
PLoS One.2021, 16(9):e0257243.
Pharmaceuticals (Basel).2021, 14(7):633.
J Biosci.2020, 45:46.
Int J Mol Sci.2019, 20(11):E2734
Invest New Drugs.2017, 35(2):166-179
Related and Featured Products
Bioprocess Biosyst Eng. 2016 Sep;39(9):1435-40.
A biotransformation process for the production of cucurbitacin B from its glycoside using a selected Streptomyces sp.[Pubmed:
27145940 ]
Cucurbitacin B (CuB) and its glycoside, Cucurbitacin B 2-O-beta-D-glucoside (CuBg), abundantly occur in the pedicels of Cucumis melo. Compared with CuB, CuBg is not efficiently extracted from the pedicels. Furthermore, the anticancer activity of CuBg is lower than that of the aglycone.
METHODS AND RESULTS:
A process for CuBg biotransformation to CuB was developed for the first time. A strain of Streptomyces species that converts CuBg into CuB was isolated from an enrichment culture of C. melo pedicels. After optimization of conditions for enzyme production and biotransformation, a maximum conversion rate of 92.6 % was obtained at a CuBg concentration of 0.25 g/L. When biotransformation was performed on C. melo pedicel extracts, the CuB concentration in the extracts increased from 1.50 to 3.27 g/L. The conversion rate was almost 100 %.
CONCLUSIONS:
The developed process may be an effective biotransformation method for industrial production CuB from C. melo pedicels for pharmaceuticals.