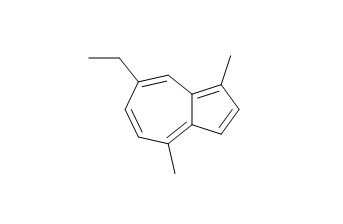
Chamazulene
Chamazulene is an important factor for the antioxidant power of chamomile oil, it presents interesting properties concerning radical processes. Chamazulene may contribute to the anti-inflammatory activity of chamomile extracts by inhibiting the leukotriene synthesis and additional antioxidative effects.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Acta Chromatographica2021, 00960.
Biomed Pharmacother.2024, 181:117647.
Chemistry of Plant Materials.2019, 215-222
Korean. J. Pestic. Sci.2024, 28(3):241-248.
Sci Rep.2018, 8(1)
Life Sci.2019, 216:259-270
Food Chem.2019, 275:746-753
Anat Rec2018, 24264
Aging (Albany NY).2021, 13(19):22867-22882.
Korean J Environ Agric.2018, 37(4):260-267
Related and Featured Products
Planta medica, 1994, 60(05):410-413.
Chamazulene: An Antioxidant-Type Inhibitor of Leukotriene B4 Formation.[Reference:
WebLink]
Matricine and its transformation product Chamazulene are constituents of chamomile extracts. Both have been demonstrated to exert anti-inflammatory activity in vivo. Since preparations from chamomile are used for the treatment of inflammatory skin and bowel diseases, we studied the effects of these compounds on the leukotriene production in neutrophilic granulocytes.
METHODS AND RESULTS:
Chamazulene inhibited the formation of leukotriene B4 in intact cells and in the 105,000 × g supernatant fraction in a concentration-dependent manner. The IC50 values were 15 and 10 µM, respectively. Matricine showed no effect up to 200 µM. Chamazulene (IC50: 2 µM), but not matricine, blocked the chemical peroxidation of arachidonic acid. Additionally, matricine (up to 200 µM) had no effects on the cyclooxygenase and 12-lipoxygenase activities in human platelets.
CONCLUSIONS:
Therefore, it is concluded that Chamazulene, but not matricine, may contribute to the anti-inflammatory activity of chamomile extracts by inhibiting the leukotriene synthesis and additional antioxidative effects.
Research Communications in Molecular Pathology & Pharmacology, 1996, 92(3):361-364.
Investigation of the effect of chamazulene on lipid peroxidation and free radical processes.[Reference:
WebLink]
Oxygen toxicity and related free radical reactions are implicated in numerous pathophysiological conditions, like atherosclerosis, inflammation, gastric ulceration, neuronal degeneration, tumour promotion. The flowers of Matricaria chamomilla, Asteraceae, have been used therapeutically for conditions in which oxidative stress is supposed to be implicated.
METHODS AND RESULTS:
We considered interesting to investigate the effect of Chamazulene, the active substance of chamomile, on free radical processes. Membrane lipid peroxidation was induced by Fe2+/ascorbate and assessed as the 2-thiobarbituric acid reactive material. The hydroxyl radical scavenging activity was studied as the competition of Chamazulene with DMSO for HO. generated by Fe3+/ascorbate. Finally, the interaction of Chamazulene with the N-centered stable free radical DPPH was estimated photometrically (517 nm). It was found that Chamazulene inhibited lipid peroxidation in a concentration and time dependent manner presenting an IC50 of 18 microM after 45 min incubation. It could also inhibit the autoxidation of DMSO (33 mM) by 76% at 25 mM, and had a weak capacity to interact with DPPH.
CONCLUSIONS:
In conclusion, Chamazulene presents interesting properties concerning radical processes.
Natural Product Research,2014, 28(24):2321-2323.
Antioxidant and radical scavenging activities of chamazulene.[Reference:
WebLink]
Essential oils (EOs) of chamomile contain several bioactive compounds, including monoterpenes, sesquiterpenes, triterpenes and fatty acids. Hydrodistillation of chamomile EO induces the formation of Chamazulene, a bioactive compound.
METHODS AND RESULTS:
Chamazulene was isolated from the EO by column chromatography. The total antioxidant capacity confirmed a higher antioxidant activity of Chamazulene (IC50 = 6.4 μg mL(- 1)) than of ascorbic acid (IC50 = 12.8 μg mL(- 1)), α-tocopherol (IC50 = 20.5 μg mL(- 1)) and of butylated hydroxytoluene (BHT) (IC50 = 30.8 μg mL(- 1)). Chamazulene was unable to react with DPPH√. However, when Chamazulene was assayed with ABTS√, a strong and significantly (P < 0.05) higher free radical scavenging activity was observed (IC50 = 3.7 μg mL(- 1)), with respect to BHT (IC50 = 6.2 μg mL(- 1)) and α-tocopherol (IC50 = 11.5 μg mL(- 1)).
CONCLUSIONS:
The results of this work show that Chamazulene is an important factor for the antioxidant power of chamomile oil.