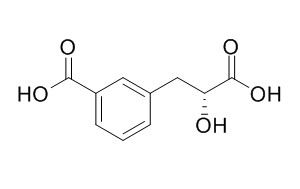
Cerberic acid B
Standard reference
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Evid Based Complement Alternat Med.2016, 2016:4357656
J Ethnopharmacol.2024, 318(Pt B):116961.
J. of The Korean Society of Food Culture2017, 144-149
Food Research International2016, 106-113
Nat Commun.2023, 14(1):8142.
J Pharm Biomed Anal.2018, 151:32-41
Journal of Research in Pharmacy.2022, 26(6):p1752-1757.
Sci Rep.2021, 11(1):11936.
Phytomedicine.2016, 23(4):331-9
Food Bioscience2023, 52:102412
Related and Featured Products
Fitoterapia. 2010 Oct;81(7):852-4.
Phenylpropionic acid derivates from the bark of Cerbera manghas.[Pubmed:
20546844]
METHODS AND RESULTS:
Two new phenylpropionic acid derivates, cerberic acid A (1) and Cerberic acid B (2), were isolated from the bark of Cerbera manghas. Their structures were established on the basis of spectroscopic methods including IR, ESI-FT-ICR-MS, 1D and 2D NMR.
CONCLUSIONS:
Primary bioassays showed that compound 1 possessed weak cytotoxic activity against HepG2, MCF-7, and HeLa cell lines with IC(50) values of 44.7, 52.3, 48.7 μg/ml, respectively.
Separation Science\s&\stechnology, 2015 , 50 (15) :2360-2366.
Separation and Purification of 15-Demethylplumieride, Cerberic Acid B, and Kaempferol-3-Rutinoside from Plumeria rubra ‘Acutifolia’ by High-Speed Counter-Current Chromatography[Reference:
WebLink]
METHODS AND RESULTS:
Three compounds, 15-demethylplumieride (I), Cerberic acid B (II), and kaempferol-3-rutinoside (III) were successfully separated from the flowers of Plumeria rubra ‘Acutifolia’ by high-speed counter-current chromatography (HSCCC) for the first time. The crude extract from Plumeria rubra ‘Acutifolia’ was treated with D101 macroporous resin and divided into two parts: fraction 1 (15% ethanol eluent) and fraction 2 (30% ethanol eluent). Two hundred and thirty milligrams of fraction 1 were separated by HSCCC with the solvent system of n -butanol-methanol-water (4:0.5:4, v/v/v), yielding 17 mg of I and 28 mg of II. One hundred and thirty-five milligrams of fraction 2 were separated by HSCCC with the solvent system of ethyl acetate- n -butanol-water (4.4:0.6:5, v/v/v), yielding 7 mg of III. The purities of the three compounds were determined by HPLC-DAD as 96.04%, 98.38%, and 97.97%, respectively.
CONCLUSIONS:
Their structures were identified by UV, MS, and NMR. The established methods were simple, fast, and convenient, which can be applied to the preparation of reference substances for bioactivity, quality control, fingerprint analysis, and other research of Plumeria rubra ‘Acutifolia’.