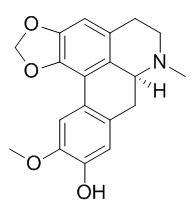
Cassythicine
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J of l. Chroma.&Related Tech2020, 43(11-12):414-423.
Food Chem.2020, 327:126992.
Int. J. of Pha. and Phy. Res.2015, 7(1):144-149
Int J Mol Sci.2018, 19(9):E2601
Front Endocrinol (Lausanne).2020, 11:568436.
Microorganisms.2021, 9(12):2514.
Molecules.2019, 24(24):E4536
Chinese Pharmacological Bulletin2019, 35(8):1120-1125
University of Limpopo2016, 1777
Bioinformatics 2024, 586957
Related and Featured Products
Molecules. 2012 Apr 5;17(4):4197-208.
(-)-Kunstleramide, a new antioxidant and cytotoxic dienamide from the bark of Beilschmiedia kunstleri gamble.[Pubmed:
22481540]
METHODS AND RESULTS:
A new dienamide, (2E,4E)-7-(3',4'-dimethoxyphenyl)-N-ethyl-6-(R)-hydroxyhepta- 2,4-dienamide, named (-)-kunstleramide (1), were isolated from the bark of Beilschmiedia kunstleri Gamble together with one neolignan: (+)-kunstlerone (2) and seven known alkaloids: (+)-nornuciferine (3), (-)-isocaryachine (4), (+)-Cassythicine (5), (+)-laurotetanine (6), (+)-boldine (7), noratherosperminine (8), (+)-N-demethylphyllocaryptine (9).
Their structures were established from spectroscopic techniques, most notably 1D- and 2D-NMR, UV, IR, OR, circular dichroism (CD) spectra and LCMS-IT-TOF.
CONCLUSIONS:
(-)-Kunstleramide (1) exhibited very poor dose-dependent inhibition of DPPH activity, with an IC₅₀ value of 179.5 ± 4.4 μg/mL, but showed a moderate cytotoxic effect on MTT assays of A375, A549, HT-29, PC-3 and WRL-68 with EC₅₀ values of 64.65, 44.74, 55.94, 73.87 and 70.95 µg/mL, respectively.
Molecules. 2011 Apr 13;16(4):3119-27.
Lancifoliaine, a new bisbenzylisoquinoline from the bark of Litsea lancifolia.[Pubmed:
21490559]
METHODS AND RESULTS:
A new bisbenzylisoquinoline, lancifoliaine (1), together with seven known alkaloids--N-allyllaurolitsine (2), reticuline (3), actinodaphnine, norboldine, pallidine, Cassythicine and boldine--were isolated from the stem bark of Litsea lancifolia (Lauraceae). In addition to that of lancifoliaine, complete ¹³C-NMR data of N-allyl-laurolitsine (2) was also reported. The alkaloidal structures were elucidated by means of high field 1D- and 2D-NMR IR, UV, and LCMS-IT-TOF spectral data.
CONCLUSIONS:
N-Allyllaurolitsine (2) showed a moderate vasorelaxant activity on isolated rat aorta.
J Nat Prod. 1998 Apr;61(4):480-4.
Antipoliovirus structure-activity relationships of some aporphine alkaloids.[Pubmed:
9584402]
METHODS AND RESULTS:
A series of 18 aporphinoids have been tested in vitro against human poliovirus. The aporphines (+)-glaucine fumarate (1), (+)-N-methyllaurotetanine (4), (+)-isoboldine (7), and (-)-nuciferine, HCl (10) were found to be active with selectivity indices > 14. The nature of the 1, 2-substituents of the isoquinoline moiety appeared to be critical for antipoliovirus activity. An SAR study demonstrated the importance of a methoxyl group at C-2 on the tetrahydroisoquinoline ring for the induction of antipoliovirus activity.
CONCLUSIONS:
Molecular modeling of some compounds in this series revealed the close similarities between the three-dimensional conformational features of the inactive 1,2-substituted derivatives (+)-boldine (6) and (+)-laurolitsine (5) with derivatives containing the 1,2-(methylenedioxy) moiety, which were generally found to be inactive as exemplified by (+)-Cassythicine (9).
Molecules. 2011 Aug 4;16(8):6582-90.
(+)-Kunstlerone, a new antioxidant neolignan from the Leaves of Beilschmiedia kunstleri gamble.[Pubmed:
21818061]
METHODS AND RESULTS:
A new neolignan, 3,4-dimethoxy-3',4'-methylenedioxy-2,9-epoxy-6,7-cyclo-1,8-neolign-11-en-5(5H)-one, which has been named (+)-kunstlerone (1), together with six known alkaloids: (+)-norboldine (2), (+)-N-methylisococlaurine (3), (+)-Cassythicine (4), (+)-laurotetanine (5), (+)-boldine (6) and (-)-pallidine (7), were isolated from the leaves of Beilschmiedia kunstleri.
The structures were established through various spectroscopic methods notably 1D- and 2D-NMR, UV, IR and LCMS-IT-TOF.
CONCLUSIONS:
(+)- Kunstlerone (1) showed a strong antioxidant activity, with an SC(50) of 20.0 µg/mL.