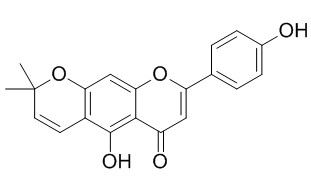
Carpachromene
Carpachromene could be a potential anti-inflammatory agent, it blocks protein expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in LPS-stimulated macrophages.Carpachromene shows significant α-glucosidase inhibitory activity. It also exhibits significant cytotoxicity against HepG2, PLC/PRF/5 and Raji cancer cell lines in vitro.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Horticulturae2024, 10(5), 486.
ACS Pharmacol. Transl. Sci.2022, 5,7,479-490
Phytomedicine.2019, 55:229-237
Food Sci Nutr.2023, 11(9):5532-5542.
Food Chem. 2020, 320:126530
Tropical J. of Pha. Research2017, 16(3):543-552
Food Res Int.2024, 197(Pt 1):115244.
Molecular & Cellular Toxicology2017, 13(3):271-278
Curr Pharm Des.2024, 30(1):71-80.
Antioxidants (Basel).2020, 9(2):E99
Related and Featured Products
J Agric Food Chem. 2013 Nov 20;61(46):11008-15.
Bioassay guided isolation and identification of anti-inflammatory active compounds from the root of Ficus formosana.[Pubmed:
24200240]
Activity-directed fractionation and purification processes were employed to identify the anti-inflammatory active compounds using lipopolysaccharide (LPS)-stimulated mouse macrophage (RAW264.7) in vitro.
METHODS AND RESULTS:
Air-dried roots of Ficus formosana were extracted with methanol and separated into n-hexane, chloroform, ethyl acetate, n-butanol, and water layers. Among them, the chloroform layer showed strong activity and was subjected to separation and purification by using various chromatographic techniques. Five compounds showing potent activity were identified by comparing spectral data to be β-sitosterol, stigmasterol, psoralen, kaempferol, Carpachromene, and syringic aldehyde. When macrophages were treated with psoralen and kaempferol together with LPS, a concentration-dependent inhibition of nitric oxide (NO) and tumor necrosis factor (TNF-α) productions were detected. Western blotting revealed that kaempferol, psoralen, and Carpachromene blocked protein expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in LPS-stimulated macrophages.
CONCLUSIONS:
The results confirmed that the traditional use of F. formosana could be a potential anti-inflammatory agent.
Mol Nutr Food Res. 2008 Dec;52(12):1530-8.
Isolation of tyrosinase inhibitors from Artocarpus heterophyllus and use of its extract as antibrowning agent.[Pubmed:
18683821 ]
A new furanoflavone, 7-(2,4-dihydroxyphenyl)-4-hydroxy-2-(2-hydroxy propan-2-yl)-2, 3-dihydrofuro(3, 2-g)chromen-5-one (artocarpfuranol, 1), together with 14 known compounds, dihydromorin (2), steppogenin (3), norartocarpetin (4), artocarpanone (5), artocarpesin (6), artocarpin (7), cycloartocarpin (8), cycloartocarpesin (9), artocarpetin (10), brosimone I (11), cudraflavone B (12), Carpachromene (13), isoartocarpesin (14), and cyanomaclurin (15) were isolated from the wood of Artocarpus heterophyllus.
METHODS AND RESULTS:
Their structures were identified by interpretation of MS,( 1)H-NMR,( 13)C-NMR, HMQC, and HMBC spectroscopic data. Among them, compounds 1-6 and 14 showed strong mushroom tyrosinase inhibitory activity with IC(50) values lower than 50 microM, more potent than kojic acid (IC(50) = 71.6 microM), a well-known tyrosinase inhibitor. In addition, extract of A. heterophyllus was evaluated for its antibrowning effect on fresh-cut apple slices. It was discovered that fresh-cut apple slices treated by dipping in solution of 0.03 or 0.05% of A. heterophyllus extract with 0.5% ascorbic acid did not undergo any substantial browning reaction after storage at room temperature for 24 h. The antibrowning effect was significantly better than samples treated with the extract (0.03 or 0.05%) or ascorbic acid (0.5%) alone.
CONCLUSIONS:
The results provide preliminary evidence supporting the potential of this natural extract as antibrowning agent in food systems.
Natural Product Communications, 2015, 10(9):1585-7.
α-Glucosidase and 15-Lipoxygenase Inhibitory Activities of Phytochemicals from Calophyllum symingtonianum.[Pubmed:
26594765]
METHODS AND RESULTS:
A phytochemical investigation of the crude extracts of the bark and leaves of Calophyllum symingtonianum has resulted in the isolation of inophyllum D, inophyllum H, calanone, isocordato-oblongic acid, amentoflavone, Carpachromene and lupenone. Their chemical structures were elucidated and confirmed by spectroscopic analysis.
CONCLUSIONS:
All flavonoids and coumarins showed significant α-glucosidase inhibitory activity, while amentoflavone gave a positive result against 15-lipoxygenase inhibition.
Planta Med. 2005 Dec;71(12):1165-7.
Cytotoxic flavonoids and new chromenes from Ficus formosana f. formosana.[Pubmed:
16395655 ]
METHODS AND RESULTS:
Two new chromenes, ficuformodiol A and ficuformodiol B, and nine known compounds including one chromene, spatheliachromen, six flavonoids, obovatin, Carpachromene ( 5), apigenin ( 6), norartocarpetin ( 7), steppogenin, 6-prenylpinocembrin, one benzopyran, chromenylacrylic acid, and one isocoumarin, ( R)-(-)-mellein, were obtained from the cytotoxic chloroform-soluble fraction of the stems of Ficus formosana f. formosana (Moraceae). Their structures were determined by means of spectroscopic analyses.
CONCLUSIONS:
Compounds 5, 6 and 7 exhibited significant cytotoxicity against HepG2, PLC/PRF/5 and Raji cancer cell lines in vitro.