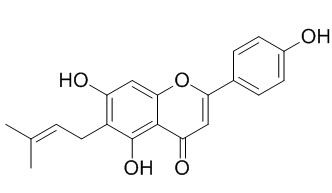
4',5,7-Trihydroxy-6-prenylflavone
6-Prenylapigenin(4',5,7-Trihydroxy-6-prenylflavone) shows potent inhibitory activity on melanin formation, it may be potential sources for skin whitening agents. It also shows moderate cytotoxicities against breast cancer (MDA-MB-435S) and lung adenocarcinoma (A549) cell lines.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Nutrients.2024, 16(16):2612.
ACS Chem Biol.2019, 14(5):873-881
J of Liquid Chromatography & Related Technologies2024, 47(1-5):14-25.
Journal of Food Hygiene and Safety2019, 34(5):413-420
Phytofrontiers2024, 2690-5442.
VNU J Science: Med.&Pharm. Sci.2024.2588-1132
Nutrients.2024, 16(20):3521.
Food Res Int.2017, 96:40-45
Front Mol Neurosci.2023, 15:1083189.
Heliyon.2024, 10(7):e28755.
Related and Featured Products
Nat. Prod. Commun., 2011, 6(9):1397-402.
Artocarpus plants as a potential source of skin whitening agents.[Pubmed:
21941923]
Artocarpus plants have been a focus of constant attention due to the potential for skin whitening agents. In the in vitro experiment, compounds from the Artocarpus plants, such as artocarpanone, norartocarpetin, artocarpesin, artogomezianol, andalasin, artocarbene, and chlorophorin showed tyrosinase inhibitory activity. Structure-activity investigations revealed that the 4-substituted resorcinol moiety in these compounds was responsible for their potent inhibitory activities on tyrosinase.
METHODS AND RESULTS:
In the in vitro assay, using B16 melanoma cells, the prenylated polyphenols isolated from Artocarpus plants, such as artocarpin, cudraflavone C, 6-prenylapigenin(4',5,7-Trihydroxy-6-prenylflavone), kuwanon C, norartocarpin, albanin A, cudraflavone B, and brosimone I showed potent inhibitory activity on melanin formation. Structure-activity investigations revealed that the introduction of an isoprenoid moiety to a non-isoprenoid-substituted polyphenol enhanced the inhibitory activity of melanin production in B16 melanoma cells. In the in vivo investigation, the extract of the wood of Artocarpus incisus and a representative isolated compound from it, artocarpin had a lightening effect on the skin of guinea pigs' backs. Other in vivo experiments using human volunteers have shown that water extract of Artocarpus lakoocha reduced the melanin formation in the skin of volunteers.
CONCLUSIONS:
These results indicate that the extracts of Artocarpus plants are potential sources for skin whitening agents.
Chem Biodivers. 2007 May;4(5):940-6.
Cytotoxic prenylated phenolic compounds from the twig bark of Garcinia xanthochymus.[Pubmed:
17511007 ]
METHODS AND RESULTS:
Three new hydroxylated xanthones with prenyl or geranyl substituents, compounds 1-3, were isolated from the twig bark of Garcinia xanthochymus, along with the four known compounds 1,4,5,6-tetrahydroxy-7,8-diprenylxanthone (4), 1,3,5,6-tetrahydroxy-4,7,8-triprenylxanthone (5), garciniaxanthone E (6), and 6-prenylapigenin (4',5,7-Trihydroxy-6-prenylflavone,7). Their structures were elucidated by extensive spectroscopic analysis, including 1D- and 2D-NMR as well as HR-MS experiments.
CONCLUSIONS:
All compounds showed moderate cytotoxicities against breast cancer (MDA-MB-435S) and lung adenocarcinoma (A549) cell lines, but lacked antifungal activity against Candida albicans.