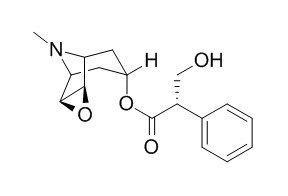
Atroscine
Atroscine has adrenolytic activity by its ability to antagonize the lethal effect of epinephrine in rats.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
BMC Complement Altern Med.2016, 16:213
Tokyo Pharmaceutical University2020, 500001431953.
Evid Based Complement Alternat Med.2017, 2017:1401279
Appl. Sci.2020, 10(8),2804
OENO One2023, 57:3.
J.Soc.Cosmet.Sci.Korea2024, 50(3): 261-270
Metabolites.2020, 10(11):440.
Agronomy2023, 13(6), 1435.
Heliyon.2023, 9(11):e21944.
Theranostics.2023, 13(9):3103-3116.
Related and Featured Products
Journal of Pharmaceutical Sciences,1966,55(3):280-284.
Adrenolytic activity of atropine, (+)-hyoscyamine, atroscine, homatropine, and related compounds[Reference:
WebLink]
METHODS AND RESULTS:
The adrenolytic activity of atropine, ( + ) and (−) hyoscyamine, (−)-hyoscine, Atroscine, and related compounds was determined by their ability to antagonize the lethal effect of epinephrine in rats. (+)-Hyoscyamine was found to be more active than atropine, and atropine was slightly more active than Atroscine (racemic hyos-cine). The levo-isomers, ( − )-hyoscyamine and (−)-hyoscine, were inactive.
These results indicate that (+)-hyoscyamine and (+)-hyoscine are responsible for the effect of atropine and Atroscine, respectively.
CONCLUSIONS:
Hornatropine and benztropine, but not tropine itself, were active. Benztropine was approximately one-fiftieth as active as phentolamine. A pair of esters of tropine had weak adrenoiytic activity, but their pseudotropine isomers were inactive. Atropine aminoxide (genatropin) and atropine methyl nitrate had no adrenolytic activity.
Eur J Pharmacol. 1983 May 20;90(1):75-83.
Structure-activity requirements for hypotension and alpha-adrenergic receptor blockade by analogues of atropine.[Pubmed:
6135619 ]
The hypotensive action of various antimuscarinic compounds structurally related to atropine was studied in conscious, unanesthetized rats.
METHODS AND RESULTS:
The alpha-adrenolytic activity of these agents was assessed both in vivo (blockade of norepinephrine-induced pressor response) and in vitro (displacement of [3H]WB-4101 binding). Benztropine, homatropine and hyoscyamine caused hypotension and produced alpha-adrenergic receptor blockade similar to atropine. Other analogues were either inactive (Atroscine, scopolamine, tropic acid and tropine) or evoked nonspecific changes in blood pressure and lacked alpha-adrenolytic activity (benactyzine, eucatropine, methylatropine, methylhomatropine and methylscopolamine).
CONCLUSIONS:
Based on these data, we propose the following structure-activity relationship for hypotension and alpha-adrenolytic activity: (a) the tropine moiety is inactive unless it is attached to another group by an ester linkage, (b) chemical modification of the tropine moiety, including quaternization, decreases potency, (c) the d-stereoisomer appears to be more potent than the corresponding 1-form.