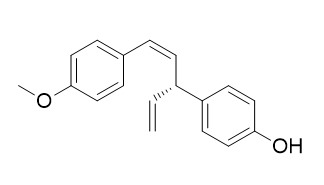
4'-O-Methylnyasol
4'-O-Methylnyasol may have potential to be developed as medicines for the treatment of allergies by inhibiting the activation of mast cells.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Curr Res Virol Sci.2022, 3:100019.
Chemistry of plant raw materials2021, 1:pp 139-150
Toxins (Basel).2021, 13(12):898.
Fitoterapia.2024, 175:105958.
Fitoterapia.2018, 124:92-102
Journal of Molecular Liquids2021, 334:116014.
Research J. Pharm. and Tech.2020, 13(7):3059-3064.
Comput Biol Med.2024, 178:108775.
Korean Herb. Med. Inf.2021, 9(2):231-239.
Curr Issues Mol Biol.2023, ;45(2):1601-1612.
Related and Featured Products
Biomed Pharmacother. 2016 Dec;84:1061-1066
Inhibitory effects of norlignans isolated from Anemarrhena asphodeloides on degranulation of rat basophilic leukemia- 2H3Cells.[Pubmed:
27780134 ]
Anemarrhena asphodeloides is known to suppress inflammation and lower various fevers.
METHODS AND RESULTS:
To determine the active component of A. asphodeloides, ethanol (EtOH) extract of A. asphodeloides rhizomes was fractionized. The compounds isolated from the dichloromethane (CH2Cl2) soluble fraction were identified as 4'-O-Methylnyasol (1), nyasol (2), 3″-methoxynyasol (3), 3″-hydroxy-4″-methoxy-4″-dehydroxynyasol (4), 4-hydroxybenzaldehyde (5), and 4-hydroxyacetophenone (6). The four norlignans (1-4) potently inhibited the release of β-hexosaminidase from immunoglobulin E (IgE)/dinitrophenol-conjugated bovine serum albumin (DNP-BSA)-treated rat basophilic leukemia (RBL)-2H3 and A23187 plus phorbol 12-myristate 13-acetate co-treated isolated rat primary mast cells, as markers of degranulation and histamine release. The intraperitoneal treatment with the EtOH extract significantly suppressed the fetal reaction, and serum histamine release induced by compound 48/80 in mice.
CONCLUSIONS:
These results suggest that the four active norlignan compounds and the EtOH extract of A. asphodeloides may have potential to be developed as medicines for the treatment of allergies by inhibiting the activation of mast cells.
J Chromatogr A. 2006 Feb 3;1104(1-2):69-74.
Preparative isolation and purification of four compounds from the Chinese medicinal herb rhizoma Anemarrhenae by high-speed counter-current chromatography.[Pubmed:
16364341]
High-speed counter-current chromatography (HSCCC) was applied to the separation and purification of mangiferin, neomangiferin, cis-hinkiresinol and (-)-4'-O-Methylnyasol from the Chinese medicinal herb rhizoma Anemarrhenae.
METHODS AND RESULTS:
Five hundred milligrams of crude extracts were separated by using n-butanol-acetic acid (1%) (1:1, v/v) as the two-phase solvent system and yielded 35.3 mg of neomangiferin and 245.4 mg of mangiferin. During this separation, cis-hinkiresinol and (-)-4'-O-Methylnyasol were still maintained in the stationary phase. The stationary phase was collected, evaporated to dryness and separated with light petroleum-ethyl acetate-methanol-water (1:1:1.2:0.8, v/v) and 1:1:1.4:0.6 (v/v) in gradient elution, which yielded 17.2 mg of cis-hinkiresinol and 12.4 mg of (-)-4'-O-Methylnyasol.
CONCLUSIONS:
The purities of mangiferin, neomangiferin, cis-hinkiresinol and (-)-4'-O-Methylnyasol were 96.3, 98.0, 97.3 and 98.2%, respectively, as determined by HPLC. The chemical structures of these components were identified by 1H NMR and 13C NMR.