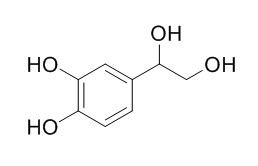
3,4-Dihydroxyphenylglycol
3,4-Dihydroxyphenylglycol has antioxidant activity, it shows a strong reactive oxygen species (ROS)-scavenging activity, reducing significantly nitrite levels with a significant decrease on iNOS expression, its phenolic derivatives could play an important role in the anti-inflammatory effect of extra virgin olive oil. Plasma 3,4-dihydroxyphenylglycol and 3-methoxy-4-hydroxyphenylglycol are insensitive indicators of alpha 2-adrenoceptor mediated regulation of norepinephrine release in healthy human volunteers.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules.2019, 24(2):E343
J Ginseng Res.2020, 44(4):611-618.
Am J Chin Med.2022, 1-20.
Sci Rep.2024, 14(1):28864.
Molecules.2021, 26(3):695.
The Korea Journal of Herbology2020, 35(3):33-45.
J Agric Food Chem.2017, 65(13):2670-2676
Chem Biol Interact.2020, 328:109200.
Foods.2023, 12(6):1130.
Front Pharmacol.2021, 12:635510.
Related and Featured Products
J Clin Pharmacol. 2015 May 22.
Pharmacodynamics of norepinephrine reuptake inhibition: Modeling the peripheral and central effects of atomoxetine, duloxetine, and edivoxetine on the biomarker 3,4-dihydroxyphenylglycol in humans.[Pubmed:
26011686]
Norepinephrine, a neurotransmitter in the autonomic sympathetic nervous system, is deaminated by monoamine oxidase to 3,4-Dihydroxyphenylglycol (DHPG).
METHODS AND RESULTS:
An indirect response model was used to describe the relationship between drug plasma concentration and DHPG concentration in plasma and CSF. The baseline plasma 3,4-Dihydroxyphenylglycol concentration (1130-1240 ng/mL) and Imax (33-37%) was similar for the 3 drugs. The unbound plasma drug IC50 (IC50U ) based on plasma 3,4-Dihydroxyphenylglycol was 0.973 nM for duloxetine, 0.136 nM for atomoxetine and 0.041 nM for edivoxetine. The baseline CSF 3,4-Dihydroxyphenylglycol concentration (1850-2260 ng/mL) was similar for the 3 drugs, but unlike plasma 3,4-Dihydroxyphenylglycol, the Imax for 3,4-Dihydroxyphenylglycol was 38% for duloxetine, 53% for atomoxetine and 75% for edivoxetine. The IC50U based on CSF 3,4-Dihydroxyphenylglycol was 2.72 nM for atomoxetine, 1.22 nM for duloxetine and 0.794 nM for edivoxetine.
CONCLUSIONS:
These modeling results provide insights into the pharmacology of NET inhibitors and the use of DHPG as a biomarker.
J Agric Food Chem. 2015 Jan 28;63(3):836-46.
Naturally occurring hydroxytyrosol derivatives: hydroxytyrosyl acetate and 3,4-dihydroxyphenylglycol modulate inflammatory response in murine peritoneal macrophages. Potential utility as new dietary supplements.[Pubmed:
25526103 ]
METHODS AND RESULTS:
This work evaluated the effects of extra virgin olive oil (EVOO) phenols, hydroxytyrosyl acetate (2) and 3,4-Dihydroxyphenylglycol (3), as well as two new acyl derivatives of 3, 4-(1,2-di(butanoyloxy)ethyl)benzene-1,2-diol (7) and 4-(1,2-di(lauroyloxy)ethyl)benzene-1,2-diol (8), on LPS-stimulated murine peritoneal macrophages in comparison with hydroxytyrosol (HTy, 1). Compounds 2, 3, 7, and 8 showed a strong reactive oxygen species (ROS)-scavenging activity, reducing significantly nitrite levels with a significant decrease on iNOS expression [2 (50 μM, 0.44 ± 0.03; 100 μM, 0.44 ± 0.01; p < 0.01); 3 (50 μM, 0.37 ± 0.03; 100 μM, 0.37 ± 0.01; p < 0.001); 7 (50 μM, 0.45 ± 0.06; p < 0.01)] . However, only 2 and 3 down-regulated COX-2 expression [2 (50 μM, 0.72 ± 0.04, p < 0.05; 100 μM, 0.54 ± 0.06, p < 0.01); 3 (50 μM, 0.56 ± 0.05, p < 0.05; 100 μM, 0.37 ± 0.04; p < 0.001)] and prevented IKBα degradation [2 (100 μM, 1.63 ± 0.14, p < 0.01); 3 (100 μM, 1.82 ± 0.09; p < 0.01)] ; the diacylated compounds 7 and 8 showed worse anti-inflammatory activity than the parent 3. In conclusion, 2 and 3 phenolic derivatives could play an important role in the anti-inflammatory effect of EVOO.
CONCLUSIONS:
The implication of this study for the nutrition and general health of the population rests in the possible use of natural HTy derivatives with better hydrophilic/lipophilic balance, thus improving its pharmacodynamic and pharmacokinetic profiles, as new dietary supplements in foods.
Mol Nutr Food Res. 2012 Jul;56(7):1137-47.
Alperujo extract, hydroxytyrosol, and 3,4-dihydroxyphenylglycol are bioavailable and have antioxidant properties in vitamin E-deficient rats--a proteomics and network analysis approach.[Pubmed:
22648667 ]
Olive products are rich in phenolic compounds, which are natural antioxidants in vitro. We tested the in vivo effects of alperujo, an olive production by-product, as well as hydroxytyrosol and 3,4-Dihydroxyphenylglycol (DHPG) isolated from alperujo, on indices and pathways of oxidative and metabolic stress in a vitamin E-deficient rat model.
METHODS AND RESULTS:
Rats were fed a vitamin E-deficient diet for 10 weeks, followed by this diet supplemented with either 100 mg/kg diet dα-tocopherol, alperujo extract, hydroxytyrosol, or 10 mg/kg diet DHPG, for a further 2 weeks. We detected alperujo phenolics in tissues and blood, indicating they are bioavailable. Alperujo extract partially ameliorated elevated plasma levels of thiobarbituric acid reactive substances and also lowered plasma cholesterol levels, whereas hydroxytyrosol increased plasma triglyceride levels. Proteomics and subsequent network analysis revealed that hepatic mitochondrial aldehyde dehydrogenase (ALDH2), of which protein and activity levels were regulated by dα-tocopherol and olive phenolics, represents a novel central regulatory protein hub affected by the dietary interventions.
CONCLUSIONS:
The in vivo free radical scavenging properties of olive phenolics appear relatively modest in our model. But alternative mechanisms, including regulation of ALDH2, may represent relevant antioxidant mechanisms by which dietary olive phenolics could have beneficial impact on cardiovascular health.
Life Sci. 1991;49(1):75-84.
Plasma 3,4-dihydroxyphenylglycol (DHPG) and 3-methoxy-4-hydroxyphenylglycol (MHPG) are insensitive indicators of alpha 2-adrenoceptor mediated regulation of norepinephrine release in healthy human volunteers.[Pubmed:
1646924]
METHODS AND RESULTS:
The usefulness of the plasma concentrations of two major metabolites of norepinephrine (NE), 3,4-Dihydroxyphenylglycol (DHPG) and 3-methoxy-4-hydroxyphenylglycol (MHPG), as indicators of neuronal NE release was investigated. The potent alpha 2-adrenoceptor agonist, dexmedetomidine, induced only about 15% maximal reductions in the metabolite concentrations, in spite of almost total inhibition of neuronal NE release, as evidenced by 90% reductions in plasma NE concentrations. Similarly, administration of the alpha 2-adrenoceptor antagonist atipamezole was followed by only small increases in plasma DHPG and no change in MHPG levels, in spite of almost six-fold, albeit short-lasting, increases in plasma NE. In contrast, a single dose of the reversible monoamine oxidase type A (MAO-A) inhibitor moclobemide reduced plasma DHPG levels by 78% and MHPG levels by 51%.
CONCLUSIONS:
It is concluded that the plasma concentrations of DHPG and MHPG are largely determined by intraneuronal, MAO-A-dependent metabolism of NE, and do not accurately reflect acute alterations in neuronal NE release. The concentration of NE in venous plasma is clearly a more sensitive indicator of alpha 2-adrenoceptor-mediated regulation of NE release.
Food Chem. 2013 Sep 1;140(1-2):154-60.
A study of the precursors of the natural antioxidant phenol 3,4-dihydroxyphenylglycol in olive oil waste.[Pubmed:
23578627]
3,4-Dihydroxyphenylglycol (DHPG) is a potent antioxidant recently found in the free form in olive oil and table olives. 3,4-Dihydroxyphenylglycol can be recovered from olive oil solid waste by a hydrothermal treatment. It was observed that an increase in the concentration of 3,4-Dihydroxyphenylglycol occurred when alperujo aqueous extracts were subjected to mild thermal conditions (post-treatment). This fact indicates that certain solubilized compounds or precursors containing 3,4-Dihydroxyphenylglycol which is released with the post-treatment.
METHODS AND RESULTS:
In the present study, the precursors of 3,4-Dihydroxyphenylglycol were identified and characterized after extraction from alperujo using thermal treatment and purification by fractionation on Amberlite® XAD16 polyamide and semi-preparative reverse-phase HPLC columns.
CONCLUSIONS:
all of which contain a 3,4-Dihydroxyphenylglycol moiety, potentially explaining the increases in the concentration of this phenolic compound in olive oil waste.