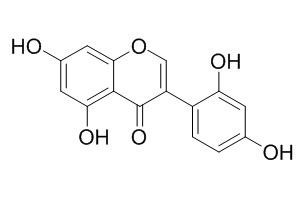
2'-Hydroxygenistein
2'-Hydroxygenistein has antifungal activity, dimerization of it causes a remarkable increase of antifungal activity. It shows significant concentration-dependent inhibitory effects on the release of beta-glucuronidase and lysozyme from rat neutrophils in response to formyl-Met-Leu-Phe/cytochalasin B. 2'-Hydroxygenistein of genistein can enhance its antioxidant activity and cell cytotoxicity in MCF-7 human breast cancer cells.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Food Chemistry: X.2022, 2022.100270
Chem Res Toxicol. 2022, acs.chemrestox.2c00049.
Braz J Med Biol Res. 2016, 49(7)
J Pharmacol Sci.2021, 147(2):184-191.
Nutrients.2020, 12(11):3448.
Srinagarind Medical Journal2017, 32(1)
Biomed Pharmacother.2022, 146:112497.
Environ Toxicol.2023, 23929.
Institute of Food Science & Technology2021, 18 December.
Tokyo Pharmaceutical University2020, 500001431953.
Related and Featured Products
Phytother Res. 2004 Feb;18(2):128-30.
Antiplasmodial constituents of Cajanus cajan.[Pubmed:
15022164]
METHODS AND RESULTS:
Bioactivity-guided fractionation of extracts of roots and leaves of Cajanus cajan afforded 8 compounds: betulinic acid, biochanin A, cajanol, genistein and 2'-Hydroxygenistein, longistylin A and C, and pinostrobin.
CONCLUSIONS:
The two stilbenes, longistylin A and C, and betulinic acid showed a moderately high in vitro activity against the chloroquine-sensitive Plasmodium falciparum strain 3D7.
Bioorg Med Chem Lett. 2004 Feb 23;14(4):1011-4.
Anti-inflammatory flavonoids and pterocarpanoid from Crotalaria pallida and C. assamica.[Pubmed:
15013012]
One new isoflavone, 5,7,4'-trihydroxy-2'-methoxyisoflavone (3) and seven, and four known compounds were isolated from the barks of Crotalaria pallida and the seeds of C. assamica, respectively.
METHODS AND RESULTS:
The known compounds, apigenin (1) and 2'-Hydroxygenistein (2), isolated from C. pallida, showed significant concentration-dependent inhibitory effects on the release of beta-glucuronidase and lysozyme from rat neutrophils in response to formyl-Met-Leu-Phe/cytochalasin B (fMLP/CB) with IC(50) values of 2.8+/-0.1 and 17.7+/-1.9, and 5.9+/-1.4 and 9.7+/-3.5 microM, respectively. The known compounds, daidzein (4) and 2'-hydroxydaidzein (6), isolated from C. pallida, inhibited of the release of lysozyme and beta-glucuronidase from rat neutrophils in response to fMLP/CB with IC(50) values of 26.3+/-5.5 and 13.7+/-2.6 microM, respectively. Compounds 1 and 4 also showed significant concentration-dependent inhibitory effects on superoxide anion generation in rat neutrophils stimulated with fMLP/CB with IC(50) values of 3.4+/-0.3 and 25.1+/-5.0 microM, respectively. Compounds 1 and 5, previously isolated from C. pallida, showed the inhibition of NO production in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages and LPS/interferon-gamma (IFN-gamma)-stimulated N9 microglial cells with IC(50) values of 10.7+/-0.1 and 13.9+/-1.1 microM, respectively.
CONCLUSIONS:
Flavonoids, suppressed chemical mediators in inflammatory cells, may have value in treatment and prevention of central and peripheral inflammatory diseases associated with excess production of chemical mediators.
J Microbiol Biotechnol. 2009 Nov;19(11):1348-54.
2'-hydroxylation of genistein enhanced antioxidant and antiproliferative activities in mcf-7 human breast cancer cells.[Pubmed:
19996686]
METHODS AND RESULTS:
Bioconversion of the isoflavonoid genistein to 2'-Hydroxygenistein (2'-HG) was performed using isoflavone 2'-hydroxylase (CYP81E1) heterologously expressed in yeast. A monohydroxylated product was analyzed by liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS) and NMR spectrometry and was identified as 2'-HG. An initial bioconversion rate of 6% was increased up to 14% under optimized conditions. After recovery, the biological activity of 2'-HG was evaluated. Bioconverted 2'-HG showed higher antioxidant activity against 1,1- diphenyl-2-picryl hydrazine (DPPH) and 2,2'-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) radicals than did genistein. Furthermore, 2'-HG exhibited greater antiproliferative effects in MCF-7 human breast cancer cells than did genistein.
CONCLUSIONS:
These results suggest that 2'- hydroxylation of genistein enhanced its antioxidant activity and cell cytotoxicity in MCF-7 human breast cancer cells.
Z Naturforsch C. 2000 Mar-Apr;55(3-4):165-74.
A new class of biflavonoids: 2'-hydroxygenistein dimers from the roots of white lupin.[Pubmed:
10817204]
METHODS AND RESULTS:
Two novel isoflavonoid dimers presumably originating from 2'-Hydroxygenistein, 5,7,4'-trihydroxycoumaranochroman-4-one-(3-->5"')-5",7",2"'4"'- tetrahydroxyisoflavone (1, lupinalbisone A) and 5,7,4'-trihydroxycoumaranochroman-4-one-(3-6")-5",7",2"',4"'-te trahydroxyisoflavone (2, lupinalbisone B) were isolated from the roots of Lupinus albus L., and their structures involving relative stereochemistry were elucidated by spectroscopic methods.
CONCLUSIONS:
Using horse radish peroxidase and 2'-Hydroxygenistein (3) as the substrate revealed the formation of these dimers together with 5,7,4'-trihydroxycoumaronochromone (4, lupinalbin A). Dimerization of 3 caused a remarkable increase of antifungal activity.