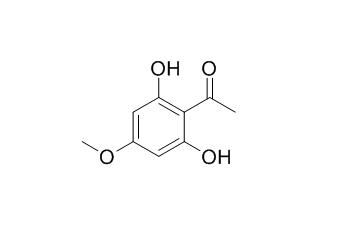
2',6'-Dihydroxy-4'-methoxyacetophenone
2',6'-Dihydroxy-4'-methoxyacetophenone is a phytoalexin after fungal inoculation with Botrytis cinerea or UV light irradiation, it has spore germination inhibition on Botrytis cinerea and Phomopsis perniciosa, with ED50 values of 45 and 410 uM, respectively. 2',6'-Dihydroxy-4'-methoxyacetophenone exhibits significant antifungal activities.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Industrial Crops and Products2022, 188:115638
Antioxidants (Basel).2021, 10(8):1300.
Phytomedicine.2023, 116:154841.
J Clin Med.2022, 11(13):3662.
Journal of Ginseng Research2021, 25 November
Plants (Basel).2024, 13(23):3314.
Emirates Journal of Food and Agriculture.2022, 34(6): 528-536.
Eur J Pharmacol.2024, 981:176883.
Drug Test Anal.2018, 10(10):1579-1589
Virus Res.2023, 335:199199.
Related and Featured Products
Phytochemistry, 1994, 35(2):331-3.
2',6'-Dihydroxy-4'-methoxyacetophenone, a phytoalexin from the roots of Sanguisorba minor[Reference:
WebLink]
METHODS AND RESULTS:
The root tissue of Sanguisorba minor produced 2',6'-Dihydroxy-4'-methoxyacetophenone as a phytoalexin after fungal inoculation with Botrytis cinerea or UV light irradiation.
CONCLUSIONS:
The ED50 of this compound on spore germination inhibition was 45 and 410 μM for Botrytis cinerea and Phomopsis perniciosa, respectively.
Journal of the Japan Wood Research Society, 1997, 43:108-111.
Antifungal activity of 2′,6′-dihydroxy-4′-methoxyacetophenone and related compounds[Reference:
WebLink]
METHODS AND RESULTS:
We investigated the relationship between the antifungal activity of 2',6'-Dihydroxy-4'-methoxyacetophenone, a component found in the leaves of Rhododendron dauricum L. and of related compounds and their chemical structures, using several common plant pathogenic fungi.
Acetophenones having hydroxyl and/or methoxyl substituents on their aromatic rings showed strong activity against all fungal species tested at a concentration of 500 μml-1. Mono-, 2′,4′-di-, 2′,5′-di-, 2′,6′-di- and 2′,4′,6′-tri-substituted acetophenones were generally more active than 3′4′-di-, 3′,5′-di-and 3′,4′,5′-tri-substituted ones. Although the hyphal growths of some fungi almost were inhibited completely by mono- and 2′,5′-di-substituted acetophenones, none of the fungi, with a few exceptions, were killed.
CONCLUSIONS:
2′,6′-Dihydroxyacetophenone and 2',6'-Dihydroxy-4'-methoxyacetophenone exhibited significant antifungal activities against all test fungi, whereas 2′-hydroxy-6′-methoxyacetophenone had limited activity. The ortho-hydroxyls to the acetyl in the molecules play an important role in antifungal activities, because methylation of the hydroxyls resulted in reduced activities. In contrast, the antifungal activities of 2′,4′-dihydroxyacetophenone and 2′,4′,6′-trihydroxyacetophenone were enhanced by the methylation of para-hydroxyl. The limited activity of 2′,4′,6′-trihydroxycetophenone suggested that one of the hydroxyls in the molecule was unfavorable for antifungal activity.
Chin J Nat Med. 2012 Jan;10(1):36-9.
Chemical constituents of Allophylus longipes.[Pubmed:
23302528 ]
To investigate the chemical constituents of Allophylus longipes.
METHODS AND RESULTS:
Compounds were isolated and purified by various chromatographic techniques and their structures were elucidated by physicochemical characteristics and spectral data. Twenty-five compounds were isolated and identified as cycloart-24-en-3β, 26-diol (1), 3-oxotrirucalla-7, 24-dien-21-oic acid (2), zizyberenalic acid (3), colubrinic acid (4), ent-4(15)-eudesmene-1β, 6α-diol (5), 4(15)-eudesmene-1β, 8α-diol (6), 4(15)-eudesmene-1β, 5α-diol (7), methyl asterrate (8), betulin (9), betulinic aldehyde (10), betulinic acid (11), 3β-hydroxy-5α, 8α-epidioxyergosta-6, 22-dien (12), 3-oxo-19α-hydroxyurs-12-en-28-oic acid (13), ursolic acid (14), scopoletin (15), fraxidin (16), cleomiscosin A (17), 4-hydroxy-3-methoxybenzaldehyde (18), 4-hydroxy-3-methoxycinnamaldehyde (19), 2',6'-Dihydroxy-4'-methoxyacetophenone (20), p-(aminoalkyl)-benzoic acid (21), 4-hydroxy-3-methoxybenzoic acid (22), 1-O-p-coumaroylglucose (23), β-sitosterol (24), and poriferast-5-ene-3β, 4β-diol (25).
CONCLUSIONS:
All the compounds were isolated from Allophylus longipes for the first time.
Verbasoside
Catalog No: CFN95015
CAS No: 61548-34-3
Price: $318/5mg
Dactylorhin A
Catalog No: CFN95032
CAS No: 256459-34-4
Price: $238/20mg
2'-Rhamnoechinacoside
Catalog No: CFN95035
CAS No: 1422390-59-7
Price: $368/10mg
3,7,25-Trihydroxycucurbita-5,23-dien-19-al
Catalog No: CFN95150
CAS No: 85372-65-2
Price: $318/5mg
Henricine
Catalog No: CFN95244
CAS No: 107783-46-0
Price: $413/5mg
13(S)-Hydroxyoctadeca-9(Z),11(E)-dienoic acid (13-HODE)
Catalog No: CFN95307
CAS No: 18104-45-5
Price: $318/10mg
2,11,12-Trihydroxy-7,20-epoxy-8,11,13-abietatriene
Catalog No: CFN95428
CAS No: 1608462-12-9
Price: $318/10mg
Kidjolanin
Catalog No: CFN95503
CAS No: 38395-01-6
Price: $318/5mg
12beta-Acetoxy-7beta-hydroxy-3,11,15,23-tetraoxo-5alpha-lanosta-8,20-dien-26-oic acid
Catalog No: CFN95515
CAS No: 1245946-62-6
Price: $318/5mg
Oxyphyllol B
Catalog No: CFN95575
CAS No: 226546-99-2
Price: Inquiry(manager@chemfaces.com)