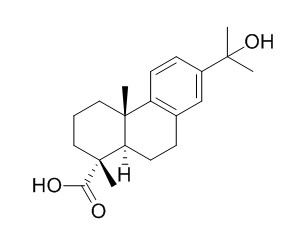
15-Hydroxydehydroabietic acid
15-Hydroxydehydroabietic acid exhibits a promising alpha-glucosidase inhibitory activity, it also shows significant antibacterial activities.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Brain Res Bull.2024, 218:111103.
Evid-Based Compl Alt2020, 7202519:13
Nutrients.2021, 13(3):978.
Jurnal Ilmu Pertanian Indonesia2023, 28(4):525-533.
J Chromatogr A.2022, 1685:463640.
Phytomedicine.2019, 57:95-104
Anim Cells Syst (Seoul).2024, 28(1):381-391.
J Ethnopharmacol.2019, 228:132-141
Journal of Physiology & Pathology in Korean Medicine.2018, 32(2): 106-112
Arch Toxicol.2017, 91(10):3225-3245
Related and Featured Products
Chembiochem. 2013 Mar 4;14(4):467-73.
Resin acid conversion with CYP105A1: an enzyme with potential for the production of pharmaceutically relevant diterpenoids.[Pubmed:
23371760]
Cytochrome P450s are very versatile enzymes with great potential for biotechnological applications because of their ability to oxidize unactivated CH bonds.
METHODS AND RESULTS:
CYP105A1 from Streptomyces griseolus was first described as a herbicide-inducible sulfonylurea hydroxylase, but it is also able to convert other substrates such as vitamin D(3) . To extend the substrate pool of this interesting enzyme further, we screened a small diterpenoid compound library and were able to show the conversion of several resin acids. Binding of abietic acid, dehydroabietic acid, and isopimaric acid to the active site was assayed, and V(max) and K(m) values were calculated. The products were analyzed by NMR spectroscopy and identified as 15-hydroxyabietic acid, 15-Hydroxydehydroabietic acid, and 15,16-epoxyisopimaric acid.
CONCLUSIONS:
As the observed products are difficult to obtain by chemical synthesis, CYP105A1 has proved to be a promising candidate for biotechnological applications that combine bioconversion and chemical synthesis to obtain functionalized resin acids.
Am Ind Hyg Assoc J. 1998 Dec;59(12):889-94.
Oxidized resin acids in aerosol derived from rosin core solder.[Pubmed:
9866169]
Exposure to rosin during a variety of uses has been associated with dermal and pulmonary sensitization. Oxidized resin acids are present in many rosin products, and have been regarded as the main sensitizing rosin compounds in cases of dermal sensitization.
METHODS AND RESULTS:
This research describes oxidized resin acids identified in aerosol produced during soldering with rosin core solder. Oxidized resin acids found were 7-oxodehydroabietic acid, 15-Hydroxydehydroabietic acid, and 7-hydroxydehydroabietic acid. The presence of oxidized compounds known to be dermal sensitizers in aerosol from rosin flux soldering supports the hypothesis that resin acid compounds are pulmonary sensitizers as previously proposed.
Changes in the composition of resin acid aerosol derived from heated rosin core solder (compared with the parent material) are described.
International Journal of Pharmacy & Pharmaceutical Sciences, 2014, 6(7): 375-8.
Biotransformation of dehydroabietic acid with microbial cell cultures and α-glucosidase inhibitory activity of resulting metabolites[Reference:
WebLink]
Dehydroabietic acid (DHA, 1), a natural occurring diterpene resin acid, is an abundant resin acid in conifers, representing a natural wood protectant. The aim of this study was to use microbial cell cultures as tools for modification of 1 in order to obtain value-added functional derivatives.
METHODS AND RESULTS:
A scaled-up biotransformation of 1 by filamentous fungus Cunninghamella elegans, Rhizopus stolonifer, Gibberella fujikuroi, and Cephalosporium aphidicola were conducted for the first time. Three hydroxylated metabolites; 1Î2-hydroxydehydroabietic acid (2); 15-Hydroxydehydroabietic acid (3); and 16-hydroxy dehydroabietic acid (4). The structure of the hydroxylated metabolites were elucidated by 1-D (1H, 13C) and 2-D NMR (COSY, HMBC, HMQC, NOESY) techniques and MS analyses.
CONCLUSIONS:
Dehydroabietic acid (1) and their transformed products 2-4 exhibited a promising alpha-glucosidase inhibitory activity. Compound 1 showed 38 times more active than the standard alpha-glucosidase inhibitor, deoxynojirimycin. Compound 1 and its transformed metabolites 2-4 also showed significant antibacterial activities.