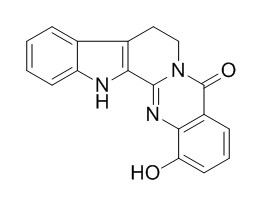
1-Hydroxyrutaecarpine
1-Hydroxyrutaecarpine exhibits cytotoxicities (ED50 values < 4 microg/mL) against P-388 or HT-29 cell lines in vitro. It exhibits antiplatelet activity induced by AA and shows an IC50 value of ca.1-2 micrograms/ml.1-Hydroxyrutaecarpine displays moderate inhibitory activity on those enzymes(Cathepsins B, L and K) at the concentration of 125 ug/ml.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Bull. Pharm. Sci., Assiut University2020, 43(2):149-155.
J Microbiol Biotechnol.2022, 32(2):141-148.
Green Chemistry2021, ISSUE 2.
Foods.2022, 11(12):1708.
J AOAC Int.2021, 104(6):1634-1651.
Plants (Basel).2021, 10(6):1192.
Exp Parasitol.2017, 183:160-166
Cardiovasc Toxicol.2021, 21(11):947-963.
J Biochem Mol Toxicol.2021, 35(5):e22731.
Molecules.2023, 28(19):6767.
Related and Featured Products
Planta Med. 2005 May;71(5):470-5.
New indolopyridoquinazoline, benzo[c]phenanthridines and cytotoxic constituents from Zanthoxylum integrifoliolum.[Pubmed:
15931588]
METHODS AND RESULTS:
Three new alkaloids, 7,8-dehydro-1-methoxyrutaecarpine, isodecarine, and 8-demethyloxychelerythrine, together with sixteen known compounds, norchelerythrine, oxychelerythrine, decarine, dihydrocherythrinylacetaldehyde, 6-acetonyldihydrochelerythrine, rutaecarpine, 1-Hydroxyrutaecarpine, gamma-fagarine, skimmianine, (-)-matairesinol, (-)-isoarctigenin, (+)-epipinoresinol, d-sesamin, lupeol, canthin-6-one, and arnottianamide have been isolated from the root bark of Zanthoxylum integrifoliolum. The structures of these new compounds were determined through spectral analyses.
CONCLUSIONS:
Among the isolates, 7,8-dehydro-1-methoxyrutaecarpine, norchelerythrine, oxychelerythrine, dihydrocherythrinylacetaldehyde, 6-acetonyldihydrochelerythrine, 1-Hydroxyrutaecarpine, gamma-fagarine, skimmianine, (-)-matairesinol, and canthin-6-one exhibited cytotoxicities (ED50 values < 4 microg/mL) against P-388 or HT-29 cell lines in vitro.
Planta Med. 1996 Apr;62(2):175-6.
Indolopyridoquinazoline alkaloids with antiplatelet aggregation activity from Zanthoxylum integrifoliolum.[Pubmed:
8657756]
METHODS AND RESULTS:
Bioassay-guided fractionation led to the isolation of three indolopyridoquinazoline alkaloids, 1-Hydroxyrutaecarpine, rutaecarpine, and 1-methoxyrutaecarpine as the active principles of antiplatelet aggregation in vitro, from the chloroform-soluble part of the fruit of Zanthoxylum integrifoliolum (Rutaceae).
CONCLUSIONS:
1-Hydroxyrutaecarpine exhibited antiplatelet activity induced by AA and showed an IC50 value of ca. 1-2 micrograms/ml.
Planta Medica, 2012 , 78 (11) :1094-1094
The alkaloid 1-hydroxyrutaecarpine inhibits cathepsin activity[Reference:
WebLink]
Cathepsins B, L and K are cysteine proteases involved in various physiological and pathological processes. CatB and L are involved in tumoral processes while catK in cases of bone resorption.
METHODS AND RESULTS:
In a search for cathepsin inhibitors we have isolated from Metrodorea stipullaris, a number of compounds, among them the alkaloid 1-Hydroxyrutaecarpine which displayed moderate inhibitory activity on those enzymes. The alkaloid showed inhibition of 79% on cathepsin B, 100% on cathepsin L and 87.5% on cathepsin K, at the concentration of 125μg/ml for the three cathepsins. IC50 experiments were run and the values obtained are described in the table below.