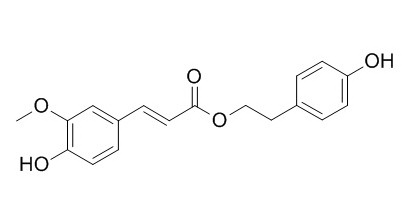
p-Hydroxyphenethyl trans-ferulate
P-Hydroxyphenethyl trans-ferulate exhibits affinity toward 5-HT(7) receptors in a competitive binding assay. P-Hydroxyphenethyl trans-ferulate can double quinone reductase specific activity in Hepa 1c1c7 cells at a level of 2.1 microg/mL (6.6 microM).
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Saudi Pharm J.2019, 27(1):145-153
Molecules.2021, 26(13):4081.
Food Quality and Safety2018, 2:213-219
Toxins (Basel).2019, 11(10):E575
Phytomedicine.2018, 47:48-57
Tea Res. Ins. Of China2017, 1-12
BMC Plant Biol.2018, 18(1):122
Acta Edulis Fungi2020, 27(02):63-76.
Mol Med Rep.2014, 9(5):1653-9
Chin. Med.J.Res. Prac.2017, 31(4)
Related and Featured Products
J Agric Food Chem. 2006 Nov 1;54(22):8417-24.
Isolation and identification of phase II enzyme-inducing agents from nonpolar extracts of green onion (Allium spp.).[Pubmed:
17061815]
The objective of the study was to isolate and identify potential cancer preventive constituents from green onion based on the ability to induce quinone reductase (QR, a representative phase II enzyme) in murine hepatoma cells (Hepa 1c1c7).
METHODS AND RESULTS:
Crude nonpolar solvent extracts were prepared from freeze-dried green onion by sequential refluxing with hexane and then ethyl acetate, followed by liquid-liquid extraction. Active fractions were subjected to the Hepa 1c1c7 bioassay-guided steps of flash chromatography, thin layer chromatography (TLC), and high-pressure preparative liquid chromatography (HPLC) to afford pure isolates capable of inducing QR. Multiple fractions were active in inducing QR.
Five pure compounds were isolated from active fractions and identified using spectroscopic methods; these were p-Hydroxyphenethyl trans-ferulate (1), 5,6-dimethyl-2-pyridinecarboxylic acid (2), ferulic acid (3), 1-(6-hydroxy-[3]pyridyl)-propan-1-one (4), and N-trans-feruloyl 3-O-methyldopamine (5).
CONCLUSIONS:
p-Hydroxyphenethyl trans-ferulate (1) doubled QR specific activity in Hepa 1c1c7 cells at a level of 2.1 microg/mL (6.6 microM).
J Nat Prod. 2006 Apr;69(4):536-41.
Serotonergic activity-guided phytochemical investigation of the roots of Angelica sinensis.[Pubmed:
16643021 ]
METHODS AND RESULTS:
Serotonin receptor (5-HT(7)) binding assay-directed fractionation of a methanol extract of the dried roots of Angelica sinensis led to the isolation and identification of 21 compounds including a new phenolic ester, angeliferulate (1), and three new phthalides, 10-angeloylbutylphthalide (2), sinaspirolide (3), and ansaspirolide (4), along with 17 known compounds, p-Hydroxyphenethyl trans-ferulate (5), Z-ligustilide (6), Z-butylidenephthalide (7), senkyunolide I (8), Z-6-hydroxy-7-methoxydihydroligustilide (9), N-butylbenzenesulfonamide (10), 11(S),16(R)-dihydroxyoctadeca-9Z,17-diene-12,14-diyn-1-yl acetate (11), (3R,8S)-falcarindiol (12), heptadeca-1-en-9,10-epoxy-4,6-diyne-3,8-diol (13), oplopandiol (14), 8-hydroxy-1-methoxy-, Z-9-heptadecene-4,6-diyn-3-one (15), imperatorin, ferulic acid, vanillin, stigmasterol, sucrose, and 1,3-dilinolenin. This is the first report of a sulfonamide (10) identified from a higher plant source, although its presence needs further investigation. Biosynthetic pathways for dimeric phthalides 3 and 4 are proposed.
CONCLUSIONS:
Compounds p-Hydroxyphenethyl trans-ferulate, 7, 11, 12, 15, and imperatorin exhibited affinity toward 5-HT(7) receptors in a competitive binding assay.