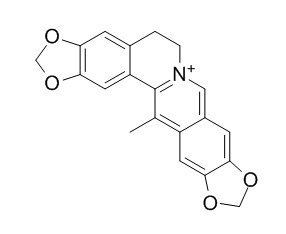
Worenine
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Nutrients.2023, 15(12):2644.
Plant Physiol Biochem.2021, 160:166-174.
Food Sci Biotechnol.2024, 34(3):611-620.
Evid Based Complement Alternat Med.2018, 2018:3610494
J Ethnopharmacol.2019, 241:112025
Life Sci.2023, 332:122107.
Front Pharmacol.2020, 11:251.
Evid Based Complement Alternat Med.2021, 8707280.
J Ethnopharmacol.2020, 254:112733.
Int. J. Mol. Sci.2022, 23(8), 4130.
Related and Featured Products
J Pharm Biomed Anal. 2010 Jan 5;51(1):236-43.
Characterization of metabolites of worenine in rat biological samples using liquid chromatography-tandem mass spectrometry.[Pubmed:
19773142]
The in vivo and in vitro metabolites of Worenine in rat were identified or characterized using a specific and sensitive liquid chromatography-tandem mass spectrometry (LC-MS/MS) method.
METHODS AND RESULTS:
In vivo samples including rat urine, feces, and plasma samples were collected after ingestion of 25 mg/kg Worenine to healthy rats. The in vivo and in vitro samples were cleaned up by a solid-phase extraction procedure (C18 cartridges) and a liquid-liquid extraction procedure, respectively. Then these pretreated samples were injected into a reversed-phase C18 column with mobile phase of methanol-ammonium acetate (2mM, adjusted to pH 3.5 with formic acid) (60:40, v/v) and detected by an on-line MS/MS system. As a result, at least twenty-seven metabolites and the parent medicine were found in rat urine after ingestion of Worenine. Seven metabolites and the parent medicine were identified or characterized in rat feces. Three metabolites and the parent medicine were detected in rat plasma. One metabolite was found in the rat intestinal flora incubation mixture, and three metabolites were characterized in the homogenized liver incubation mixture.
CONCLUSIONS:
The main phase I metabolism of Worenine in rat was dehydrogenization, hydrogenation, hydroxylation, and demethylene reactions, and that of phase II was sulfation and glucuronidation.