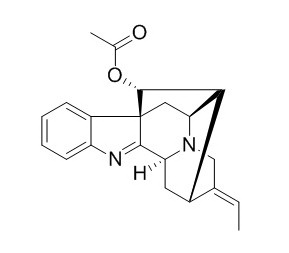
Vinorine
Vinorine is a direct biosynthetic precursor along the complex pathway to the monoterpenoid indole alkaloid ajmaline, an antiarrhythmic drug from the Indian medicinal plant Rauvolfia serpentina.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Sep Sci.2023, 46(16):e2300160.
Mol Biol Rep.2024, 51(1):117.
Molecules.2019, 24(2):329
Molecules.2020, 25(15):3353.
Functional Ecology2020, doi: 10.1111.
bioRxiv - Molecular Biology2023, 535548.
Journal of Functional Foods2023, 104:105542
J Formos Med Assoc.2020, S0929-6646(20)30425-3
Malaysian Journal of Analytical Sciences2022, 26(2):360-369.
Molecules.2019, 24(7):E1290
Related and Featured Products
Bioorg Med Chem. 2004 May 15;12(10):2787-95.
Acetyltransfer in natural product biosynthesis--functional cloning and molecular analysis of vinorine synthase.[Pubmed:
15110860]
Vinorine synthase (EC 2.3.1.160) catalyses the acetyl-CoA- or CoA-dependent reversible formation of the alkaloids Vinorine (or 11-methoxy-Vinorine) and 16-epi-vellosimine (or gardneral). The forward reaction leads to Vinorine, which is a direct biosynthetic precursor along the complex pathway to the monoterpenoid indole alkaloid ajmaline, an antiarrhythmic drug from the Indian medicinal plant Rauvolfia serpentina.
METHODS AND RESULTS:
Based on partial peptide sequences a cDNA clone was isolated and functionally expressed in Escherichia coli. The Km values of the native enzyme for gardneral and acetyl-CoA were determined to be 7.5 and 57 microM. The amino acid sequence of Vinorine synthase has highest level of identity (28-31%) to that of Papaver salutaridinol acetyltransferase, Fragaria alcohol acyltransferase, and Catharanthus deacetylvindoline acetyltransferase involved in morphine, flavor, and vindoline biosynthesis, respectively. Vinorine synthase is a novel member of the BAHD superfamily of acyltransferases.
Site-directed mutagenesis of 13 amino acid residues provided clear evidence that both, His160 and Asp164 of the consensus sequence HxxxD belong to the catalytic center. The mutations also showed that an amino acid triad is not characteristic of Vinorine synthase.
CONCLUSIONS:
The experiments demonstrated the importance of the conserved motif SxL/I/VD near the N-terminus and the consensus sequence DFGWG near the C-terminal.