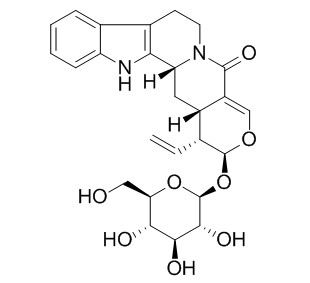
Vincosamide
Vincosamide can effect relaxation of the supercoiled pBR322 plasmid DNA in the presence of Cu2+.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Plos One.2019, 15(2):e0220084
Biomol Ther (Seoul).2024, 32(2):214-223.
J Ethnopharmacol.2017, 206:327-336
Appl. Sci.2022, 12(4), 2032.
J Ethnopharmacol.2023, 309:116302.
Antioxidants (Basel).2022, 11(8):1471.
Free Radic Biol Med.2017, 112:191-199
J Chromatogr B Analyt Technol Biomed Life Sci.2022, 1203:123307.
Cancers (Basel).2021, 13(17):4327.
Comput Biol Med.2024, 178:108775.
Related and Featured Products
Nat Prod Res. 2013 Mar;27(4-5):402-11.
The major indole alkaloid N,β-D-glucopyranosyl vincosamide from leaves of Psychotria leiocarpa Cham. & Schltdl. is not an antifeedant but shows broad antioxidant activity.[Pubmed:
22891663]
METHODS AND RESULTS:
N,β-D-glucopyranosyl Vincosamide (GPV), a major alkaloid of Psychotria leiocarpa, constitutes up to 2.5% of the dry weight in leaves. Alkaloid content was not elicited by mechanical wounding or jasmonate. At concentrations found in natural conditions or 2.5 fold higher, GPV did not inhibit herbivory in two unrelated generalist models (Helix aspersa and Spodoptera frugiperda) or in a specific interaction model (Heliconius erato fed with Passiflora suberosa). In situ staining assay showed quenching activity of hydrogen peroxide by GPV. Exposure of P. leiocarpa to acute UV-B stress did not change GPV or chlorophyll content, indicating high tolerance to this stress by the species.
CONCLUSIONS:
In vitro antioxidant tests against singlet oxygen, superoxide anions and hydroxyl radicals showed efficient quenching activity of the alkaloid. GPV was not effective as antifeedant, but it may act indirectly in P. leiocarpa protection against oxidative stress generated upon wounding, UV exposure and perhaps other environmental stresses.
Bioorg Med Chem Lett. 2013 Feb 15;23(4):959-62.
Cardioprotective potential of N,α-L-rhamnopyranosyl vincosamide, an indole alkaloid, isolated from the leaves of Moringa oleifera in isoproterenol induced cardiotoxic rats: in vivo and in vitro studies.[Pubmed:
23321560]
Hitherto unknown protective effect of N,α-L-rhamnopyranosyl Vincosamide (VR), isolated from Moringa oleifera leaves in isoproterenol (ISO)-induced cardiac toxicity was evaluated in rats.
METHODS AND RESULTS:
Oral administration of Hitherto unknown protective effect of N,α-L-rhamnopyranosyl Vincosamide (VR), isolated from Moringa oleifera leaves in isoproterenol (ISO)-induced cardiac toxicity was evaluated in rats. Oral administration of N,α-L-rhamnopyranosyl Vincosamide at 40 mg/kg for 7 days markedly reduced the ISO-induced increase in the levels of serum cardiac markers such as troponin-T, creatine kinase-MB, lactate dehydrogenase and glutamate pyruvate transaminase as well as cardiac lipid peroxidation with a parallel increase in the cellular antioxidants suggesting its cardio-protective and free radical scavenging potential, which was latter confirmed by in vitro study.
CONCLUSIONS:
Rats treated with test compound also improved the ISO-induced abnormal changes in ECG as well as in cardiac histology.
Planta Med. 2011 Feb;77(3):284-6.
Monoterpenoid indole alkaloids mediating DNA strand scission from Turpinia arguta.[Pubmed:
20717879 ]
METHODS AND RESULTS:
Two new monoterpenoid indole alkaloid derivatives, turpiniside (1) and 11-methoxyjavaniside (2), along with the known alkaloids, Vincosamide (3), (3 R)-pumiloside (4), and paratunamide C (5), were isolated from the leaves of Turpinia arguta (Lindl.) Seem. Their structures were determined on the basis of spectroscopic data.
CONCLUSIONS:
Compounds 1 and 3-5 were found to effect relaxation of the supercoiled pBR322 plasmid DNA in the presence of Cu²+.