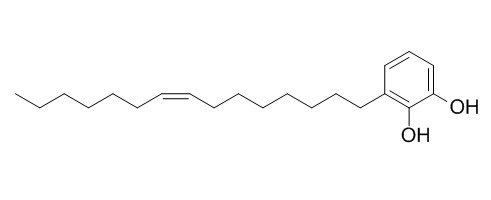
Urushiol (15:1)
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Separations2023, 10(2), 131.
Molecules.2022, 27(7):2116.
World J Mens Health.2019, 10.5534
Journal of Physiology & Pathology in Korean Medicine.2018, 32(2): 106-112
Foods.2023, 12(2):318.
Planta Med.2019, 85(4):347-355
Immunopharmacol Immunotoxicol.2024, 46(4):496-508.
Int J Mol Sci.2018, 19(9):E2825
Molecules.2018, 23(12):E3103
Acta Pharm Sin B.2024, 14(4):1772-1786.
Related and Featured Products
Chemistry & Physics of Lipids, 2002, 120(1-2):101-108.
A synthesis of the phenolic lipid, 3-[(Z)-pentadec-8-enyl] catechol, (15:1)-urushiol.[Reference:
WebLink]
METHODS AND RESULTS:
A synthesis of (15:1)-urushiol( Urushiol (15:1)), urushiol monoene, 3-[(Z)-pentadec-8-enyl] catechol, 1,2-dihydroxy-3-[(Z)-pentadec-8-enyl] benzene, one of the toxic principles of Rhus toxicodendron and of Rhus vernicifera is described. 6-Chlorohexan-1-ol protected at the OH group with ethyl vinyl ether reacted with 2,3-dimethoxybenzaldehyde in the presence of lithium to give, after removal of the protective group with methanolic 4-toluenesulphonic acid, 1-(2,3-dimethoxyphenyl) heptane-1,7-diol. Catalytic hydrogenolysis in ethanol with palladium–carbon selectively afforded 7-(2,3-dimethoxyphenyl)heptane-1-ol accompanied by a small proportion of the 7-(3-methoxyphenyl)heptane-1-diol, formed by demethoxylation. Reaction of the dimethoxy compound with boron tribromide resulted in both bromination and demethylation to give 7-(2,3-dihydroxyphenyl) heptylbromide.
CONCLUSIONS:
This bromide in tetrahydrofuran (THF) containing hexamethylphosphoric triamide reacted with excess lithium oct-1-yne to give 3-(pentadec-8-enyl)catechol which, by catalytic hydrogenation in ethyl acetate containing quinoline, selectively formed the required cis product, 3-[(Z)-pentadec-8-enyl]catechol which was identical chromatographically and spectroscopically with urushiol monoene separated from the natural product.