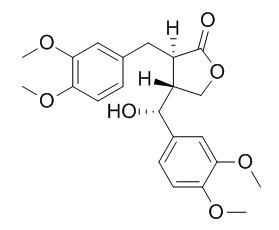
Tupichilignan A
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
TCI CO.2019, US20190151281A1
Arch Toxicol.2017, 91(10):3225-3245
FASEB J.2022, 36(7):e22387.
Food Chem.2020, 327:126992.
Sci Rep.2017, 7:46299
Preprints2017, 2017120176
J Pharm Anal.2016, 6(6):363-373
Plant Biotechnol (Tokyo).2024, 41(3):267-276.
Egyptian Pharmaceutical Journal2024, epj_205_23.
J Clin Med.2019, 8(10):E1664
Related and Featured Products
Asian Journal of Organic Chemistry, 2017,6(8).
First asymmetric total synthesis of tupichilignan A using donor‐acceptor cyclopropanes: a structural revision of tupichilignan A.[Reference:
WebLink]
METHODS AND RESULTS:
The first asymmetric total synthesis of Tupichilignan A was achieved using enantioenriched donor-acceptor cyclopropanes. The key steps include an asymmetric cyclopropanation using the Hayashi-Jørgensen catalyst, an oxy-homo-Michael reaction of a bicyclic D-A cyclopropane, an α-benzylation of a γ-lactone, a decarboxylation to furnish a trans-α,β−dibenzyl-γ-lactone, a configurational inversion of a hydroxy chiral center via oxidation, and a final reduction, all of which occur with high stereoselectivity.
CONCLUSIONS:
The spectral data of the diastereomer previously identified in the literature as Tupichilignan A were found to be inconsistent with the reported data for the natural product. On the basis of the spectral data of both synthesized diastereomers, the structure of Tupichilignan A was revised and the absolute configuration of the 7-position of Tupichilignan A was changed from R to S.