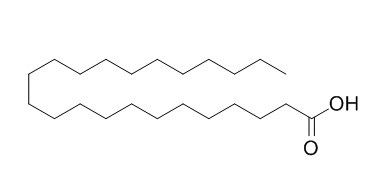
Tricosanoic acid
Tricosanoic acid is a long-chain fatty acid and shown to be a hair growth stimulant. Tricosanoic acid (23:0) could as internal standards in the quantitation of eicosapentaenoic (EPA) and docosahexaenoic (DHA) fatty acids.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Mol Med.2020, 45(5):1514-1524.
Processes2024, 12(8), 1563
Clin Exp Pharmacol Physiol.2020, doi: 10.1111
New Zealand J. Forestry Sci.2014, 44:17
Industrial Crops and Products2021, 163:113313.
Analytical Letters.2020, doi 10.1008
Redox Rep.2024, 29(1):2392329.
Front Cell Dev Biol.2021, 9:638174.
J Med Food.2024, 27(8):728-739.
Phytochem Anal.2021, 32(6):970-981.
Related and Featured Products
J Chromatogr. 1990 Nov 30;533:1-10.
Nervonic acid versus tricosanoic acid as internal standards in quantitative gas chromatographic analyses of fish oil longer-chain n-3 polyunsaturated fatty acid methyl esters.[Pubmed:
2150519]
Tricosanoic acid (23:0) and cis-15-tetracosenoic acid (nervonic acid, 24:1 n-9) were compared as choices suitable for use as internal standards in the quantitation of eicosapentaenoic (EPA) and docosahexaenoic (DHA) fatty acids.
METHODS AND RESULTS:
Experiments conducted included: (a) comparison of the flame ionisation detector responses of the two fatty acid methyl esters; (b) estimation of accurately weighed quantities of EPA and DHA using both 23:0 and 24:1 separately as internal standard; (c) determination of EPA and DHA contents of commercially available fish oil ethyl ester capsules using the two as internal standard. The results suggest that both 23:0 and 24:1 methyl esters behaved similarly in the flame ionization detector of the gas chromatograph and are comparable internal standards for use in quantitation of EPA and DHA. This includes the analysis of ethyl ester mixtures as long as interesterification of sample with solvent methanol is complete. The relatively poor solubility of the saturated 23:0 is countered by its greater stability. A possible drawback of 24:1 could be the presence of more than one positional isomer in either a 24:1n-9 standard or in the actual sample.
CONCLUSIONS:
In principle any fatty acid could serve as an internal standard as long as the limitations involved in the use of each are taken into account.
Chemistry. 2007;13(11):3150-9.
Structures of the high-temperature solid phases of the odd-numbered fatty acids from tridecanoic acid to tricosanoic acid.[Pubmed:
17212366]
Crystal structures of the high-temperature phases of odd-numbered fatty acids (C(n)H(2n-1)OOH) from tridecanoic acid (C(13)H(25)OOH) to Tricosanoic acid (C(23)H(45)OOH) are presented in this article.
METHODS AND RESULTS:
They have been determined from high-quality X-ray powder-diffraction patterns. Two types of high-temperature phases are adopted: one monoclinic A2/a with Z=8 for the fatty acids with n=13 and n=15, denoted as C'', and one monoclinic P2(1)/a with Z=4 for the longer-chain fatty acids, denoted as C'. It appears that the packing arrangement of the alkyl chains and of the carboxyl groups is similar in all of the structures. However, the arrangement at the methyl-group interface differs between the C' and C'' forms.
CONCLUSIONS:
A survey of the intermolecular interactions involved in these polymorphs coupled with a study of the effects of temperature on the structures have led us to a better understanding of the arrangement of the molecules within the high-temperature solid phases of odd-numbered fatty acids.
3,3',4',5,6,7,8-heptamethoxyflavone
Catalog No: CFN95021
CAS No: 1178-24-1
Price: $268/10mg
Lappaol A
Catalog No: CFN95067
CAS No: 62333-08-8
Price: $333/10mg
(3R,5S,E)-1,7-Diphenylhept-1-ene-3,5-diol
Catalog No: CFN95227
CAS No: 232261-31-3
Price: $318/5mg
Safflospermidine A
Catalog No: CFN95255
CAS No: 1111082-13-3
Price: $413/5mg
9-Octadecenedioic acid
Catalog No: CFN95301
CAS No: 4494-16-0
Price: $100/20mg
Batatasin IV
Catalog No: CFN95319
CAS No: 60347-67-3
Price: $318/5mg
Arvenin III
Catalog No: CFN95327
CAS No: 65597-45-7
Price: $318/5mg
Eschweilenol C
Catalog No: CFN95363
CAS No: 211371-02-7
Price: $318/10mg
Ganosinensic acid C
Catalog No: CFN95567
CAS No: 2231756-23-1
Price: $413/5mg
Irigenin 3'-O-glucoside
Catalog No: CFN95578
CAS No: N/A
Price: $318/5mg