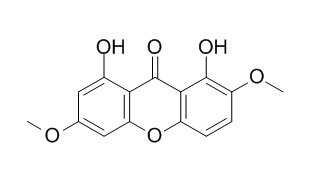
Swertiaperennin
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Biomed Pharmacother.2021, 139:111585.
Heliyon2022, 8(2):e08866.
Oxid Med Cell Longev.2020, 2020:8887251.
Front Pharmacol.2019, 10:1025
Heliyon.2024, 10(23):e40758.
Cardiovasc Toxicol.2021, 21(11):947-963.
Phytochemistry Letters2017, 449-455
Biomed Pharmacother.2024, 176:116765.
Drug Des Devel Ther.2020, 14:969-976.
Antioxidants.2022, 11(3):592.
Related and Featured Products
Planta Med. 2003 Aug;69(8):770-2.
The xanthones gentiacaulein and gentiakochianin are responsible for the vasodilator action of the roots of Gentiana kochiana.[Pubmed:
14531031]
Gentiana kochiana Perr. et Song. (Gentianaceae), a plant used in the traditional medicine of Tuscany (Italy) as antihypertensive remedy, exerts a vasodilator action on in vitro aortic rings that is probably linked to the blocking of the ryanodine-sensitive Ca++ channels.
METHODS AND RESULTS:
In the present study, three known xanthones were isolated from the crude methanolic extract of the roots: gentiacaulein, gentiakochianin, and Swertiaperennin. The first two showed a vasorelaxing activity in rat aortic preparations, pre-contracted by 3 microM norepinephrine (pIC50 = 5.00 +/- 0.032 for gentiacaulein, pIC50 = 4.95 +/- 0.068 for gentiakochianin), 20 mM KCl (pIC50 = 4.90 +/- 0.15 for gentiacaulein; 4.59 +/- 0.069 for gentiakochianin), or 5 mM caffeine; on the contrary, in the same conditions, Swertiaperennin did not show any vasodilator effect.
CONCLUSIONS:
In conclusion, gentiacaulein and gentiakochianin seem to be the compounds responsible for the vasorelaxing properties of the crude extract of Gentiana kochiana roots.
Records of Natural Products, 2016, 10(3):287-293.
Antiplasmodial Activity and Cytotoxicity of Isolated Compound from the Stem Bark of Anthocleistaliebrechtsiana[Reference:
WebLink]
METHODS AND RESULTS:
One new cerebroside derivative, namely liebrechtsianoside A (1), along with five known compounds: tetracosanoic acid (2), Swertiaperennin (3), decussatin (4), swertianin (5) and β-sitosterol glucoside (6) were isolated from the the stem bark of Anthocleista liebrechtsiana. Their structures were elucidated by interpretation of NMR and MS data, and by comparison of these data with those reported in literature.
CONCLUSIONS:
Compound 1 showed the highest antiplasmodial activity against Dd2 chloroquine-resistant strain of Plasmodium falciparum.