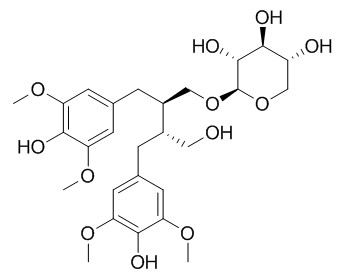
Ssioriside
Ssioriside shows moderate antioxidant activity against 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Phytochemistry Letters2017, 449-455
Sci Rep. 2017, 12953(7)
Molecules.2024, 29(17):4034.
Front Pharmacol.2021, 12:635510.
Plant J.2021, 107(6):1711-1723.
Anticancer Res.2024, 44(3):1033-1044.
The Korea Journal of Herbology2020, 35(3):33-45.
Front Pharmacol.2021, 12:762829.
Horticulture Research2020, 7:111.
Int J Mol Sci.2018, 19(9):E2825
Related and Featured Products
Nat.Prod.Sci., 2005, 11(2):97-102.
The chemical constituents and their antioxidant activity of the stem of Rhododendron mucronulatum[Reference:
WebLink]
METHODS AND RESULTS:
From the n-BuOH soluble fraction of the 70% aqueous acetone extract of Rhododendron mucronulatum stem, twelve compounds were isolated. On the basis of spectral data, they were identified as scopoletin (1), (+)-taxifolin (2), quercetin (3), (-)-catechin (4), (+)-epicatechin (5), scopolin (6), lyoniside (7), Ssioriside (8), fraxin (9), (+)-lyoniresinol-3α-O-β-D- glucopyranoside (10), (+)-taxifolin-3-O-α-L-arabinopyranoside (11), and astragalin (12), respectively. All isolated compounds were tested antioxidant activity against 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical.
CONCLUSIONS:
Compounds 2 and 3 showed the potent antioxidant activity, and compounds 5, 8, and 11 showed moderate activity.
Planta Med. 2011 Mar;77(5):441-9.
Epifriedelanol from the root bark of Ulmus davidiana inhibits cellular senescence in human primary cells.[Pubmed:
21049397]
Since cellular senescence involves organismal aging as well as diverse diseases, aging intervention might contribute to inhibit the aging process as well as aging-associated diseases.
METHODS AND RESULTS:
We tried to search for effective compounds from the root bark of ULMUS DAVIDIANA that are able to inhibit cellular senescence in human fibroblasts (HDFs) and human umbilical vein endothelial cells (HUVECs). Twenty-two compounds from the root bark of U. DAVIDIANA were isolated and screened for their inhibitory effects on adriamycin-induced cellular senescence by measuring senescence-associated β-galatosidase (SA- β-gal) activity. Among twenty-two compounds isolated, epifriedelanol (3), Ssioriside (15), and catechin-7-O- β-D-glucopyranoside (22) had inhibitory effects on adriamycin-induced cellular senescence in HDFs. Friedelin (2), epifriedelanol (3), and catechin-7-O- β-apiofuranoside (18) were active in HUVECs. In particular, epifriedelanol (3) suppressed adriamycin-induced cellular senescence as well as replicative senescence in HDFs and HUVECs.
CONCLUSIONS:
These results suggest that epifriedelanol (3) reduces cellular senescence in human primary cells and might be used to develop dietary supplements or cosmetics that modulate tissue aging or aging-associated diseases.
Zhongguo Zhong Yao Za Zhi. 2013 Jun;38(11):1740-6.
[Lignans from Machilus robusta].[Pubmed:
24010288]
Eighteen lignans were isolated from an ethanol extract of Machilus robusta by a combination of various chromatographic techniques including column chromatography over silica gel, Sephadex LH-20 and reversed-phase HPLC.
METHODS AND RESULTS:
Their structures were identified by spectroscopic data analysis as isolariciresinol-9'-O-beta-D-xylopyranoside (1), (+)-5'-methoxy-isolariciresinol-9'-O-beta-D-xylopyranoside(2), lyoniresinol-9'-O-beta-D-xylopyranoside(3), (+)-(8S, 8'S) -4, 4'-dihydroxy-3, 3', 5, 5'-tetramethoxylignan-9, 9'-diol 9-O-beta-D-xylopyranoside (Ssioriside, 4), lyoniresinol (5), meso-dihydroguaiaretic acid (6), (+)-(8S, 8'R)-3', 4, 4'-trihydroxy-3'-methoxylignan (7), (8S, 8'R)-4'-hydroxy-3, 3', 4-trimethoxylignan (meso-monomethyl dihydroguaiaretic acid, 8), (+)-guaiacin (9), isoguaiacin (10), (-)-(7'R, 8R, 8'R)-4, 4'-dihydroxy-3, 3', 5-trimethoxy-2, 7'-cyclolignan (11), henricine B (12), (-)-(7S, 7'S, 8R, 8'R)-4, 4'-dihydroxy-3, 3', 5, 5'-tetramethoxy-7, 7'-epoxylignan-9, 9'-dio] (7S, 7'S, 8R, 8'R-icariol A2, 13), (+)-(7R, 8R, 7'E)-4-hydroxy-3, 5'-dimethoxy-7, 4'-epoxy-8, 3'-neolignan-7'-ene (licarin A, 14), nectandrin B (15), machilin-I (16), (-)-pinoresinol (17), and (-)-syringaresinol (18).
CONCLUSIONS:
All compounds were isolated from this plant for the first time.
In the preliminary assay, compound 17 showed inhibitory activity against NO secretion of mouse peritoneal macrophages with an inhibition rate of 72.2% at 10 micromol x L(-1).